Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need the separation scheme for this lab. I included the examples given. EXPERIMENT 3D 3D Use of Extraction to Isolate a Neutral Compound from
I need the separation scheme for this lab. I included the examples given. 



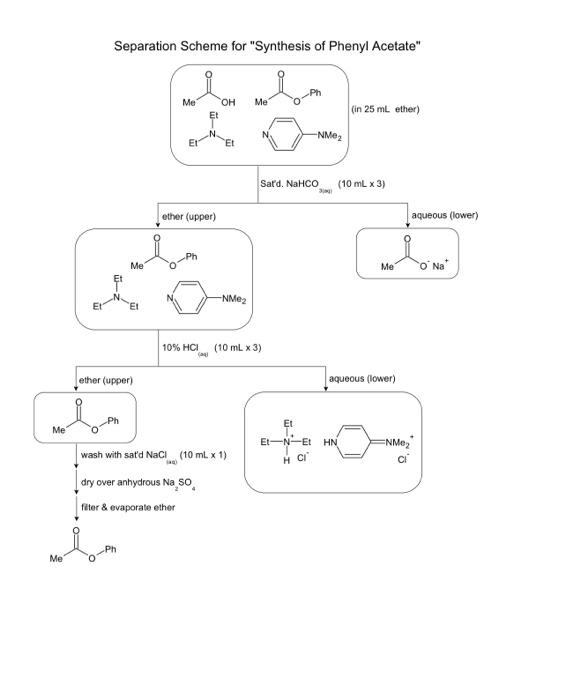
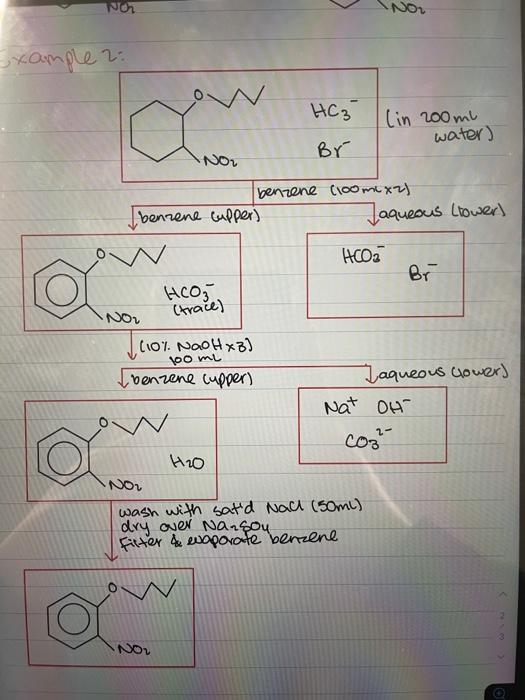
EXPERIMENT 3D 3D Use of Extraction to Isolate a Neutral Compound from a Mixture Containing an Acid or Base Impurity In this experiment, you will be given a solid sample containing an unknown neutral com- pound and an acid or base impurity. The goal is to remove the acid or base by extrac- tion and isolate the neutral compound. By determining the melting point of the neutral The three mixtures will likely be (1) water and n-butyl chloride, (2) water and n-butyl bromide, and (3) n-butyl bromide and saturated aqueous sodium bromide. 23 Experiment 3D Use of Extraction to solate a Neutral Compound compound, you will identity it from a list of possible compounds. There are many organic reactions in which the desired product, a neutral compound, is contaminated by an acid or base impurity. This experiment illustrates how extraction is used to isolate the product in such a situation In Technique 10. "Solubility you learned that organic acids and bases can become ions in acid-base reactions (see Section 10-2 B Solutions in Which the Solute fornizes and Dissociates"). Before reading on, review this material if necessary . Using this principle, you can separate an acid or base impurity from a neutral compound. The following scheme, which shows how both an acid and a base impunty are removed from the desired product illustrates how this is accomplished: 0 R-C- Neutral compound R-C-OH R-NH Acid Base impurity impunity Dissolved in other Add NaOHa. Aqueous layer Ether layer R-C-RR-NH R-C-ON Eher layer Add HC Aquitous layer R- R-NH'a Flowchart showing how acid and base impunities are removed from the desired product. The neutral compound can now be isolated by removing the water dissolved in the ether and evaporating the ether. Because other dissolves a relatively large quantity of water (15% the water must be removed in two steps. In the first step the other solution is mixed with a saturated aqueous NaCl solution. Most of the water in the ether layer will be transferred to the aqueous layer in this step (see Technique 12. Section 1291. Finally, the remainder of the wator is removed by drying the other layer over anhydrous sodium sulfate. The neutral com pound can then be isolated by evaporating the other in most organic experiments that use a separation scheme such as this, it would be necessary to perform a crystallization step to purity the neutral compound. In this experiment, however the neutral compound should be sufficiently pure at this point to identity it by melting point The organic solvent used in this experiment is ther. Recall that the full name for other is diethyl ether. Because ether is less dense than water this experiment will give you prac tice in performing extractions where the nonpolar solvent is less dense than water The following procedure provides instruction on removing an acid impurity from a neu- tral compound and isolating the neutral compound it contains an additional step that is not normally part of this kind of separation scheme: The aqueous layers from each extraction are action to Basic Laboratory Techniques segregated and acidified with aqueous HCL The purpose of this step is to verify that the acid impurity has been removed completely from the other layer. In the Optional Exercise, the sample contains a neutral compound with a base impurity, however, a detailed procedure is not given. If you are assigned this exercise, you must create a procedure by using the princ- ples discussed in this introduction and by studying the following procedure for isolating the neutral compound from an acid impunity Isolating a Neutral Compound from a Mixture Containing an Acid Impurity. Add 0.36g of an un known mixture to a screw.cap centrifuge tube * Add 10.0 mL of ether to the tube and cap it. Shake the tube until all the solid dissolves completely. Transfer this solution to a 125ml separatory funnel Add 50 mL of 10 M NaOH to the separatory funnel and shake for 30 seconds, using the same procedure descrbed in Part A. Let the layers separate. Remove the bottom (aqueous) layer and place this in an Erlenmeyer flask labeled "1 st NaOH extract Add another 5.0 mL portion of 1.0 M NaOH to the funnel and shake for 30 seconds. When the layers have sepa rated, remove the aqueous layer and put it in an Erlenmeyer flask labeled "2nd NaOH extract." While stirring, add 6 M HCl dropwise to each of the two test flasks containing the NaOH extracts until the mixtures are acidic Test the mixtures with litmus or pH paper to determine when they are acidic Observe the amount of precipitate that forms. What is the precipitate? Does the amount of precipitate in each flask indicate that all the acid impurity has been removed from the ether layer containing the unknown neutral compound? The drying procedure for an other layer requires the following additional step, which is not included in the procedure for drying a mothylene chloride layer (see Technique 12. Section 12.9. "Saturated Salt Solution"). To the ether layer in the separatory funnel, add 5.0 mL of saturated aqueous sodium chloride Shake for 30 seconds and let the layers sepa rate. Remove and discard the aqueous layer. Pour the other layer (without any water from the top of the separatory funnel into a dean, dry Erlenmeyer flask. Now dry the ether layer over granular anhydrous sodium sulfate (see Technique 12. Section 12.9. "Drying Procedure with Anhydrous Sodium Sulfate"). Complete steps 1-3 in A Macroscale Drying Procedure Step 4 is described in the next paragraph of this experiment Transfer the dred other solution with a clean, dry Pasteur pipet to a dry, preweighed Erlenmeyer flask, leaving the drying agent behind. Evaporate the other by heating the flask in a warm water bath. This should be done in a hood and can be accomplished more rapidly if a stream of dry ait or nitrogen gas is directed at the surface of the liquid (see Technique 7 Section 101. When the solvent has evaporated, remove the flask from the bath and dry the outside of the flask. Once the flask has cooled to room temperature, weigh it to determine the amount of solid solute that was in the ether layer. Obtain the melting point of the solid and identity it from the following table: Melting Point (o Fluorenone 82-85 Fluorene 116-117 1.2.4, 5-Tetrachlorobenzene 139-142 Triphenylmethanol 162-164 The minute contains 0.245 of one of the neutral compounds given in the following table and 0.12 g of benzoic acid, the acid impurity See footnote 3 2. Report the melting point (as a range!) and the mass of the neutral compound that you isolated. melting point: 141.5 -143.4 mass: 0.2155 g 3. Based on the melting point, what is the identity of this compound? 1, 2, 4,5-Tetrachlorobenzene Melting point : 139 - 142 4. Calculate the percent recovery for the neutral compound. List possible sources of loss. 0.21552 0.35789 x 100% = 60.2 % Separation Scheme for "Synthesis of Phenyl Acetate" Me Me OH (in 25 mL ether) N N -NMez Et Satd. NaHCO (10 mL x 3) ether (upper) aqueous (lower) Ph Me Me O Na -NMez 10% HCI (10 mL x 3) ether (upper) aqueous (lower) Me 20- HN Et-N-Et " ENMez wash with sat'd NaCl (10 ml x 1) dry over anhydrous Na So fter & evaporate ether Me NO NOR example 2: HC3 (in 200ml water) Br NOL benzene (100 mx2) Ibenzene cupper) Jaqueous lower) Hoa Br Hco3 (trace) AXB) boomi NO Com NaOHx benzene cupper) Jaqueous clowers Nat oH com- H2O NO wash with sat'd Nach (50mL) dry over Nansoy filter & waporate benzene NO 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


