Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need the solution for (v) and (vi) only Consider a chemical process to produce benzene at a rate of 9500kg/hr with product benzene (purity
i need the solution for (v) and (vi) only 
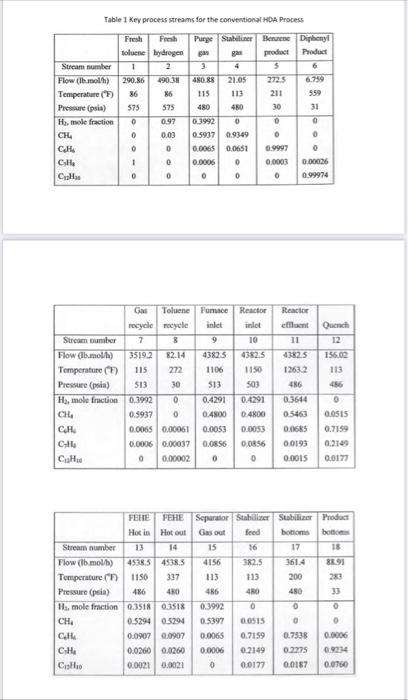
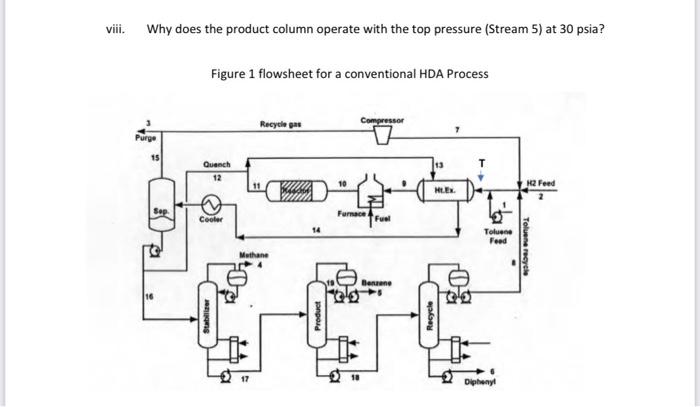
Consider a chemical process to produce benzene at a rate of 9500kg/hr with product benzene (purity 99.7\%). A process based on hydroalkylation (HAD) is considered. Figure 1 shows a simplified PFD diagram for HDA process. The basic unit operations of this process: reactor, furnace, vapor liquid separator, recycle compressor, two heat exchangers and three distillation columns. Two raw materials, hydrogen and toluene, are converted into benzene (main product), with methane and diphenyl produced as by products. The two vapor-phase reactions are: C7H8+H22C6H6C6H6+CH4(1)C12H10+H2(2) The kinetic rate expressions are functions of the partial pressure (in psia) of toluene PTon, hydrogen PH, benzene PBz and diphenyl PDp, with an Arrhenius dependence and given as r1=3.6858106exp(T25,616)PToPH0.5Hrxn=21,5001bBtur2=5.987104exp(T25,616)PBZ22.553106exp(T25,616)PDpPHHrxn=0 Where r1 and r2 have units of lbmol/min.ft3 and T(K). The flowsheet and key process streams for the process is given in Figure 1 and Table 1. i. Evaluate the equilibrium conversion at 1150F. ii. If the amount of hydrogen in the feed to the reactor is reduced to the stoichiometric amount determine the effect on the equilibrium conversion at 1150F. iii. What is the effect of reducing temperature from 1150F to 750F on the reaction rate, and reactor size? comment on your answer. iv. Determine the effect of reducing reaction pressure (to atmospheric) on the reaction rate? comment on your answer. v. It was decided to reduce the pressure of stream 3 prior to venting to atmosphere. Use heuristics 41 and 42 to determine whether or not an expander should be put in place, or a simple valve? vi. Determine the temperature of the feed entering the heat exchanger ( Ht.Ex.) (assume 7 psi pressure drop across the exchanger). Do you consider the heat integration for this exchanger "good or or poor" one. vii. Why does the stabilizer operate at 113F and 480 psia? Table 1Kev process streams for the conventional HbA Process ii. Why does the product column operate with the top pressure (Stream 5 ) at 30 psia? Figure 1 flowsheet for a conventional HDA Process Consider a chemical process to produce benzene at a rate of 9500kg/hr with product benzene (purity 99.7\%). A process based on hydroalkylation (HAD) is considered. Figure 1 shows a simplified PFD diagram for HDA process. The basic unit operations of this process: reactor, furnace, vapor liquid separator, recycle compressor, two heat exchangers and three distillation columns. Two raw materials, hydrogen and toluene, are converted into benzene (main product), with methane and diphenyl produced as by products. The two vapor-phase reactions are: C7H8+H22C6H6C6H6+CH4(1)C12H10+H2(2) The kinetic rate expressions are functions of the partial pressure (in psia) of toluene PTon, hydrogen PH, benzene PBz and diphenyl PDp, with an Arrhenius dependence and given as r1=3.6858106exp(T25,616)PToPH0.5Hrxn=21,5001bBtur2=5.987104exp(T25,616)PBZ22.553106exp(T25,616)PDpPHHrxn=0 Where r1 and r2 have units of lbmol/min.ft3 and T(K). The flowsheet and key process streams for the process is given in Figure 1 and Table 1. i. Evaluate the equilibrium conversion at 1150F. ii. If the amount of hydrogen in the feed to the reactor is reduced to the stoichiometric amount determine the effect on the equilibrium conversion at 1150F. iii. What is the effect of reducing temperature from 1150F to 750F on the reaction rate, and reactor size? comment on your answer. iv. Determine the effect of reducing reaction pressure (to atmospheric) on the reaction rate? comment on your answer. v. It was decided to reduce the pressure of stream 3 prior to venting to atmosphere. Use heuristics 41 and 42 to determine whether or not an expander should be put in place, or a simple valve? vi. Determine the temperature of the feed entering the heat exchanger ( Ht.Ex.) (assume 7 psi pressure drop across the exchanger). Do you consider the heat integration for this exchanger "good or or poor" one. vii. Why does the stabilizer operate at 113F and 480 psia? Table 1Kev process streams for the conventional HbA Process ii. Why does the product column operate with the top pressure (Stream 5 ) at 30 psia? Figure 1 flowsheet for a conventional HDA Process 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


