Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need to complete the table Make 250mL250mM iron chloride (FeCl3) stock solution, you will prepare five standard solutions with various concentrations of iron (ie.
I need to complete the table 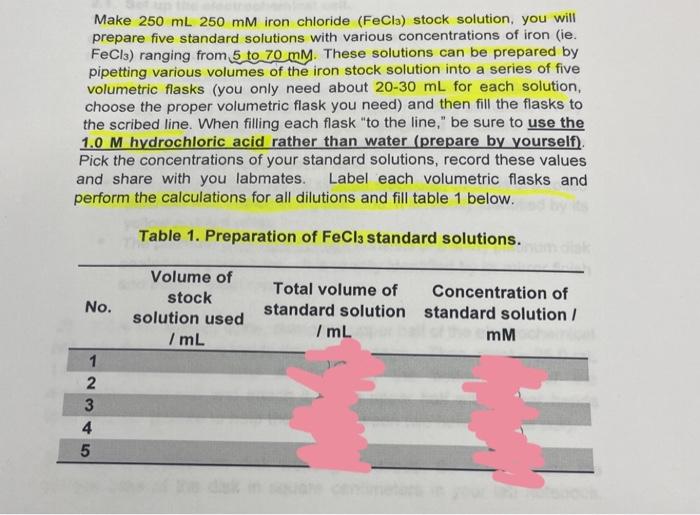
Make 250mL250mM iron chloride (FeCl3) stock solution, you will prepare five standard solutions with various concentrations of iron (ie. FeCl3 ) ranging from 5 to 70mM. These solutions can be prepared by pipetting various volumes of the iron stock solution into a series of five volumetric flasks (you only need about 20-30 mL for each solution, choose the proper volumetric flask you need) and then fill the flasks to the scribed line. When filling each flask "to the line," be sure to use the 1.0 M hydrochloric acid rather than water (prepare by yourself). Pick the concentrations of your standard solutions, record these values and share with you labmates. Label each volumetric flasks and perform the calculations for all dilutions and fill table 1 below. Table 1. Preparation of FeCl3 standard solutions 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


