Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need to see the step by step of the calculation for initial reaction rate and rate constant how do I fill in the following
I need to see the step by step of the calculation for initial reaction rate and rate constant how do I fill in the following table in part using run
here's some data:
The temperature in the lab was deg C
Part
Remember the room temperature run should be copied from run of part
At deg C the solution took s to turn blue.
At deg C the solution took s to turn blue.
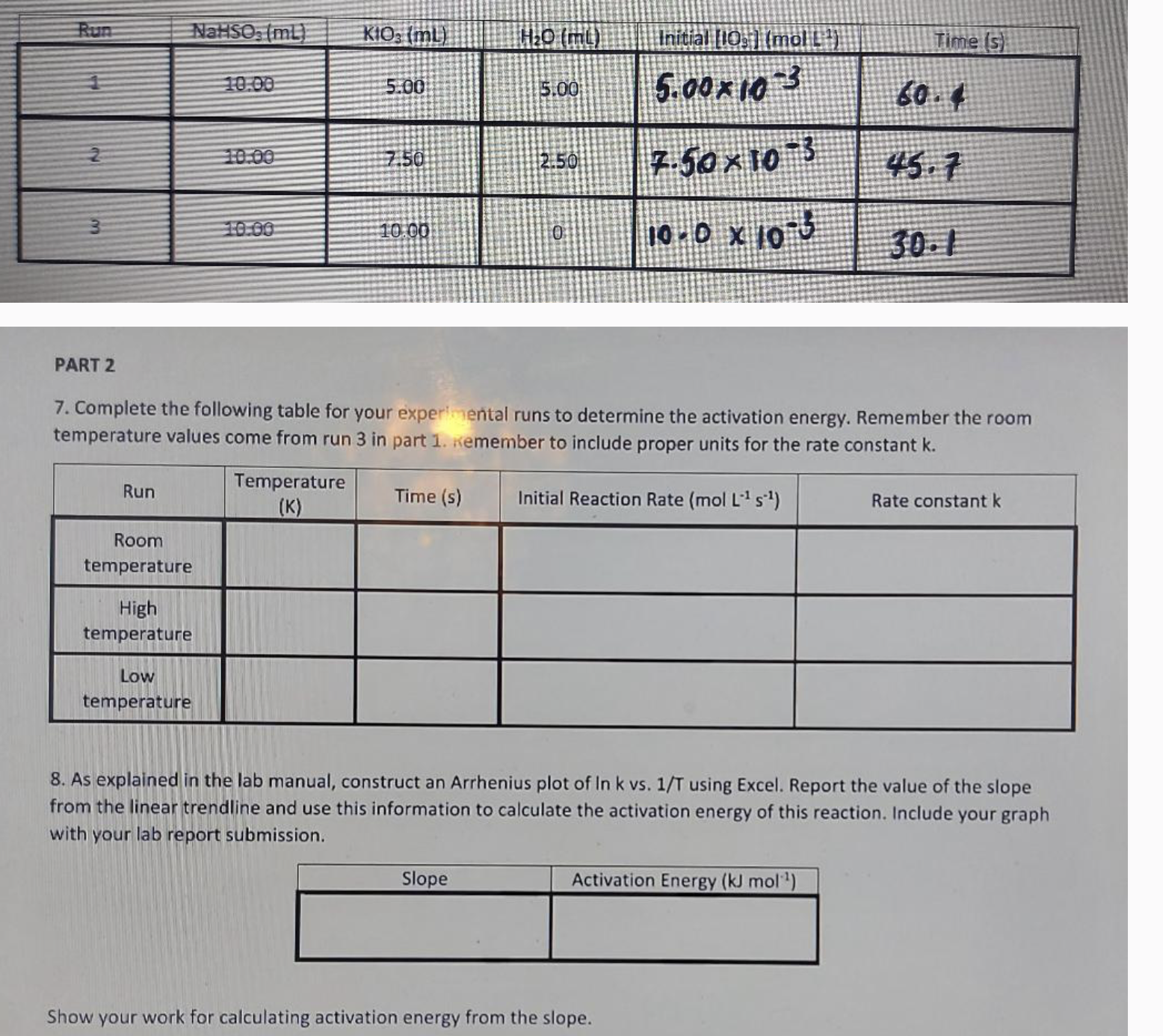
Run PART 2 NaHSO, (mL) Run Room temperature High temperature 10.00 Low temperature 10.00 10.00 KIO3 (ml) Temperature (K) 5.00 7.50 10.00 Time (s) HO (mt) 5.00 7. Complete the following table for your experimental runs to determine the activation energy. Remember the room temperature values come from run 3 in part 1. Remember to include proper units for the rate constant k. Initial Reaction Rate (mol L- s) Slope 2.50 0 Initial [10] (mol [] 5.0010 3 7.5010 3 10.0 x 105 Time (s) Activation Energy (kJ mol ) Show your work for calculating activation energy from the slope. 60.4 45.7 8. As explained in the lab manual, construct an Arrhenius plot of In k vs. 1/T using Excel. Report the value of the slope from the linear trendline and use this information to calculate the activation energy of this reaction. Include your graph with your lab report submission. Rate constant k
Step by Step Solution
★★★★★
3.38 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Step2 Explanation 6Calculate the modified rate constant k using the report...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


