Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I only need part a.) for this question. In my studying, I've managed to get to the composition of the distillate, which is 0.968, and
I only need part a.) for this question. In my studying, I've managed to get to the composition of the distillate, which is 0.968, and the feed, which is 459.25 kmol/hr, but I need help on the rest of part a.), I just don't understand how I get the composition of the bottoms.
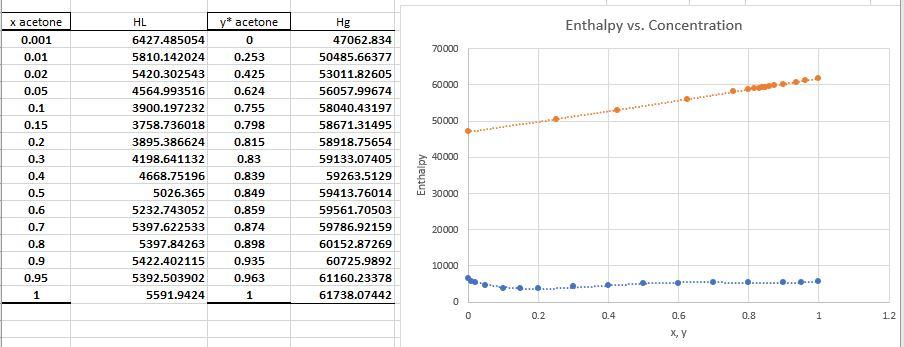
 The enthalpy graph is below:
The enthalpy graph is below:
So far, I've used Xd=(99/58.08)/((99/58.08)+(1/18.02)) to get Xd, and then F=(0.25 * 10,000)/58.08 + (0.75*10,000)/18.02 to get F=459.25 kmol/hr.
9.10 An acetone-water solution containing 25 wt% acetone is to be fractionated at a rate of 10 000 kg/h at 1 std atm pressure, and it is planned to recover 99.5% of the acetone in the distillate at a concentration of 99 wt %. The feed will be available at 26.7C (80F) and will be preheated by heat exchange with the residue product from the fractionator, which will in turn be cooled to 51.7C (125F). The distilled vapors will be condensed and cooled to 37.8C (125F) by cooling water entering at 26.7C and leaving at 40.6C. Reflux will be returned at 37.8C, at a reflux ratio L/D = 1.8. Open steam, available at 70 kN/m2, will be used at the base of the tower. The tower will be insulated to reduce heat losses to negligible values. Physical property data are given in Prob. 9.4. Determinc: (a) The hourly rate and composition of distillate and reflux. HL y* acetone 0 Enthalpy vs. Concentration 70000 60000 50000 x acetone 0.001 0.01 0.02 0.05 0.1 0.15 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 0.95 1 6427.485054 5810.142024 5420.302543 4564.993516 3900.197232 3758.736018 3895.386624 4198.641132 4668.75196 5026.365 5232.743052 5397.622533 5397.84263 5422.402115 5392.503902 5591.9424 40000 Hg 47062.834 50485.66377 53011.82605 56057.99674 58040.43197 58671.31495 58918.75654 59133.07405 59263.5129 59413.76014 59561.70503 59786.92159 60152.87269 60725.9892 61160.23378 61738.07442 0.253 0.425 0.624 0.755 0.798 0.815 0.83 0.839 0.849 0.859 0.874 0.898 0.935 0.963 1 Enthalpy 30000 20000 10000 0 0.2 0.4 0.6 0.8 1 12 X,YStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


