I placed all the examples for reference
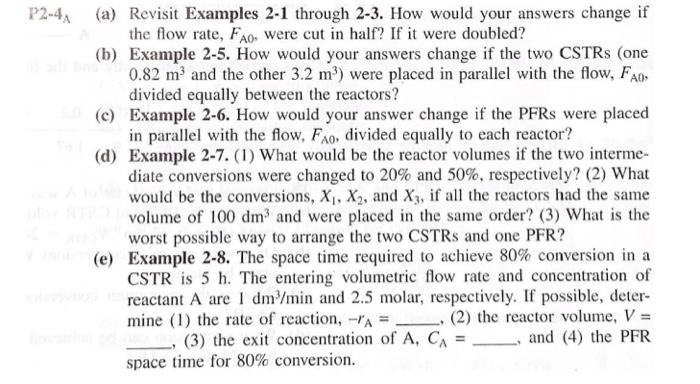
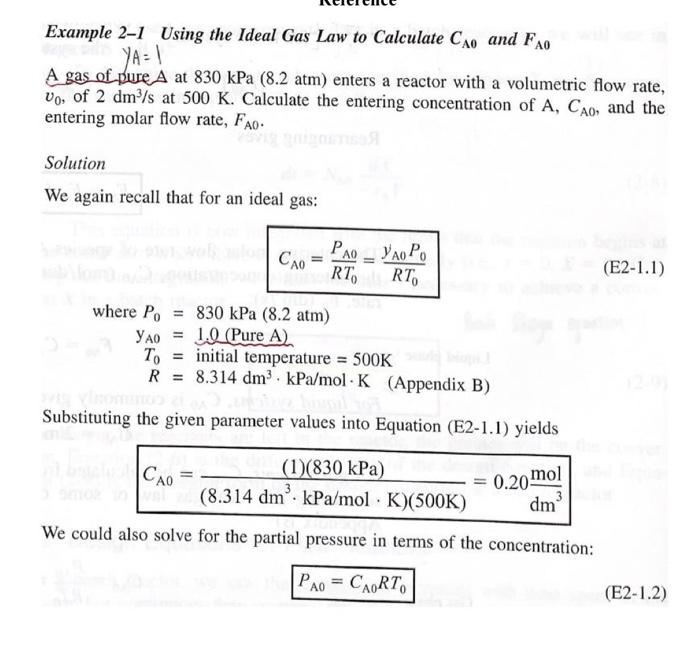
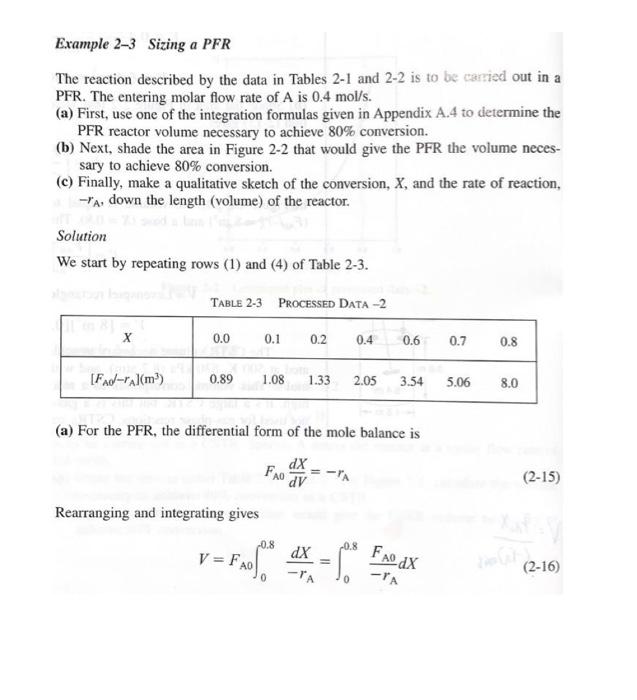
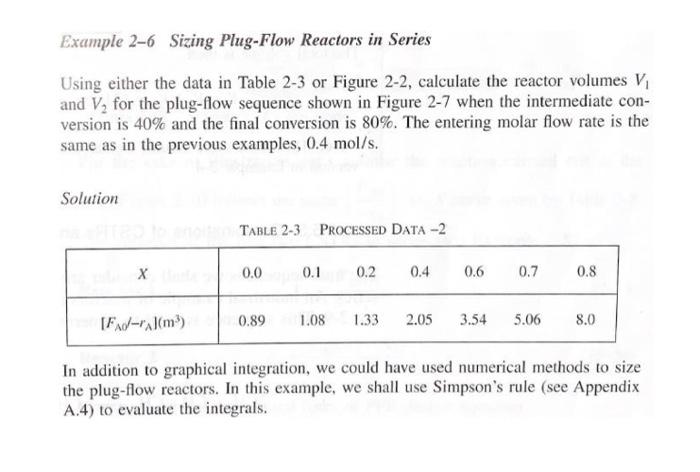
P2-4(a) Revisit Examples 2-1 through 2-3. How would your answers change if the flow rate, Fao, were cut in half? If it were doubled? (b) Example 2-5. How would your answers change if the two CSTRs (one 0.82 m and the other 3.2 m) were placed in parallel with the flow, Fo, divided equally between the reactors? (c) Example 2-6. How would your answer change if the PFRs were placed in parallel with the flow, Fao, divided equally to each reactor? (d) Example 2-7. (1) What would be the reactor volumes if the two interme- diate conversions were changed to 20% and 50%, respectively? (2) What would be the conversions, X, X2, and X3, if all the reactors had the same volume of 100 dm and were placed in the same order? (3) What is the worst possible way to arrange the two CSTRs and one PFR? (e) Example 2-8. The space time required to achieve 80% conversion in a CSTR is 5 h. The entering volumetric flow rate and concentration of reactant A are 1 dm /min and 2.5 molar, respectively. If possible, deter- mine (1) the rate of reaction, -"A = (2) the reactor volume, V = (3) the exit concentration of A, CA = and (4) the PFR space time for 80% conversion. Example 2-1 Using the Ideal Gas Law to Calculate CAo and FAO YA = 1 A gas of pure A at 830 kPa (8.2 atm) enters a reactor with a volumetric flow rate, Vo, of 2 dm?/s at 500 K. Calculate the entering concentration of A, CAO, and the entering molar flow rate, Fao. Solution We again recall that for an ideal gas: PAO YAO Po CA0 = RT RT, (E2-1.1) where P. 830 kPa (8.2 atm) 0 1.2 (Pure A) = initial temperature = 500K R = 8.314 dm kPa/mol - K (Appendix B) Substituting the given parameter values into Equation (E2-1.1) yields CA0 (1)(830 kPa) (8.314 dm. kPa/mol K)(500K) 0.20 mol dm We could also solve for the partial pressure in terms of the concentration: PAO = CART (E2-1.2) Example 2-3 Sizing a PFR The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is 0.4 mol/s. (a) First, use one of the integration formulas given in Appendix A.4 to determine the PFR reactor volume necessary to achieve 80% conversion. (b) Next, shade the area in Figure 2-2 that would give the PFR the volume neces- sary to achieve 80% conversion. (c) Finally, make a qualitative sketch of the conversion, X, and the rate of reaction, -A, down the length (volume) of the reactor. Solution We start by repeating rows (1) and (4) of Table 2-3. TABLE 2-3 PROCESSED DATA-2 0.0 0.1 0.2 0.4 0.6 0.7 0.8 [FA-ral(m) 0.89 1.08 1.33 2.05 3.54 5.06 8.0 (a) For the PFR, the differential form of the mole balance is dX FA DV (2-15) Rearranging and integrating gives 0.8 -0.8 V = FAO " - FAO X dX PA = (2-16) 0 -7A Example 2-5 Comparing Volumes for CSTRs in Series For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conver- sion of the entering species A? TABLE 2-3 PROCESSED DATA-2 0.0 0.1 0.2 0.4 0.6 0.7 0.8 [FA-rl(m) 0.89 1.09 1.33 2.05 3.54 5.06 8.0 Solution For reactor 1, we observe from either Table 2-3 or Figure E2-5.1 that when X = 0.4, then FAO ( = 2.05 m --PAL X=0.4 then FAO FAO V = TAX 0 For reactor 2, when X2 = 0.8, then =CEMO) * =**).X;= (2.05)(0.4) = 0.82 m= 820 dm x )m (F40 1: = (**)X X) V2 = (8.0 m)(0.8 -0.4) = 3.2 m = 3200 dm? = 8.0 m Ax=0.8 FAO (2-24) V2 = 3200 dm (liters) Example 2-6 Sizing Plug-Flow Reactors in Series Using either the data in Table 2-3 or Figure 2-2, calculate the reactor volumes V and V, for the plug-flow sequence shown in Figure 2-7 when the intermediate con- version is 40% and the final conversion is 80%. The entering molar flow rate is the same as in the previous examples, 0.4 mol/s. Solution Table 2-3 PROCESSED DATA-2 0.0 0.1 0.2 0.4 0.6 0.7 0.8 [FX-r(m) 0.89 1.08 1.33 2.05 3.54 5.06 8.0 In addition to graphical integration, we could have used numerical methods to size the plug-flow reactors. In this example, we shall use Simpson's rule (see Appendix A.4) to evaluate the integrals. Example 2-7 An Adiabatic Liquid-Phase Isomerization The isomerization of butane n -CH 2 -CH10 was carried out adiabatically in the liquid phase and the data in Table E2-7.1 were obtained. (Example 8.4 shows how the data in Table E2-7.1 were generated.) TARLA E2-7.1 RAW DATA X 0.0 0.2 0.4 0.6 0.65 - (kmom. h) 39 53 59 38 25 Example 2-8 Reactor Space Times and Space Velocities Calculate the space time, t, and space velocities for each of the reactors in Exam- ples 2-2 and 2-3. Solution From Example 2-1, we recall the entering volumetric flow rate was given as 2 dm's (0.002 m/s), and we calculated the concentration and molar flow rates for the conditions given to be no = 0.2 mol/dm and Fro = 0.4 mol/s. From Example 2-2, the CSTR volume was 6,4 m and the corresponding space time and space velocity are V 6.4 m 3200 s =0.89 h U 0.002 m/s SV 1.125 h 0.89 h From Example 2-3, the PFR volume was 2.165 m, and the corresponding space time and space velocity are V - 2.165 m 1083 s=0.30 h V, 0.002 m/s