Answered step by step
Verified Expert Solution
Question
1 Approved Answer
15. While casting a silver ornament, a silver-47-8 smith pours 0.12 kg of molten silver at 1010C into a 2.40-kg iron mold at a
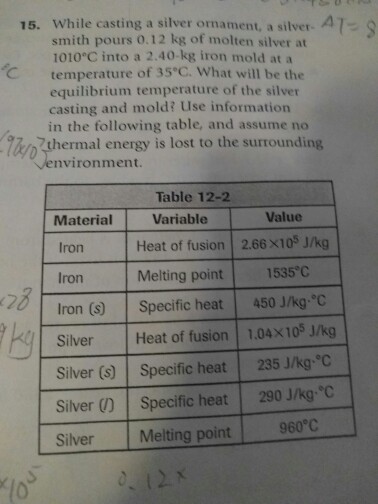
15. While casting a silver ornament, a silver-47-8 smith pours 0.12 kg of molten silver at 1010C into a 2.40-kg iron mold at a temperature of 35C. What will be the equilibrium temperature of the silver casting and mold? Use information in the following table, and assume no thermal energy is lost to the surrounding environment. 'C '90x/0 128 ky Material Iron Iron Iron (s) Silver Silver (s) Silver (1) Silver Table 12-2 Variable Heat of fusion Melting point Specific heat Heat of fusion Specific heat Specific heat Melting point 0. 12X Value 2.66X105 J/kg 1535C 450 J/kg-C 1.04X105 J/kg 235 J/kg-C 290 J/kg-C 960C
Step by Step Solution
★★★★★
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The mass of the silver is m 012kg Initial temperature of the silver is T 1010C The amount of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


