Question
What is the Ht concentration for an aqueous solution with pOH = 4.27 at 25 C? Express your answer to two significant figures and
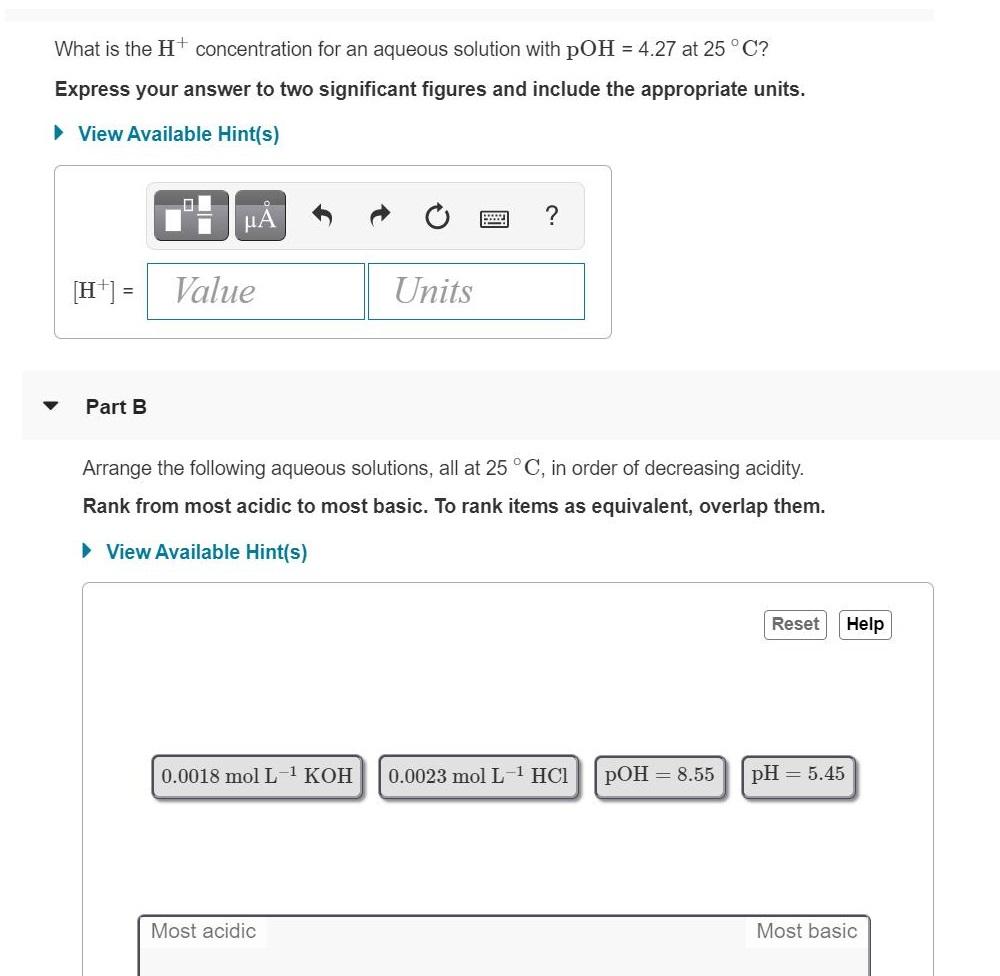
What is the Ht concentration for an aqueous solution with pOH = 4.27 at 25 C? Express your answer to two significant figures and include the appropriate units. View Available Hint(s) [H*] = Value Units Part B Arrange the following aqueous solutions, all at 25 C, in order of decreasing acidity. Rank from most acidic to most basic. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help 0.0018 mol L1 KOH 0.0023 mol L-1 HCl pOH = 8.55 pH = 5.45 Most acidic Most basic
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



