Question
2- What is the conjugate acid of HPO3- ? Express your answer as a chemical formula. View Available Hint(s) B ? Part D Among
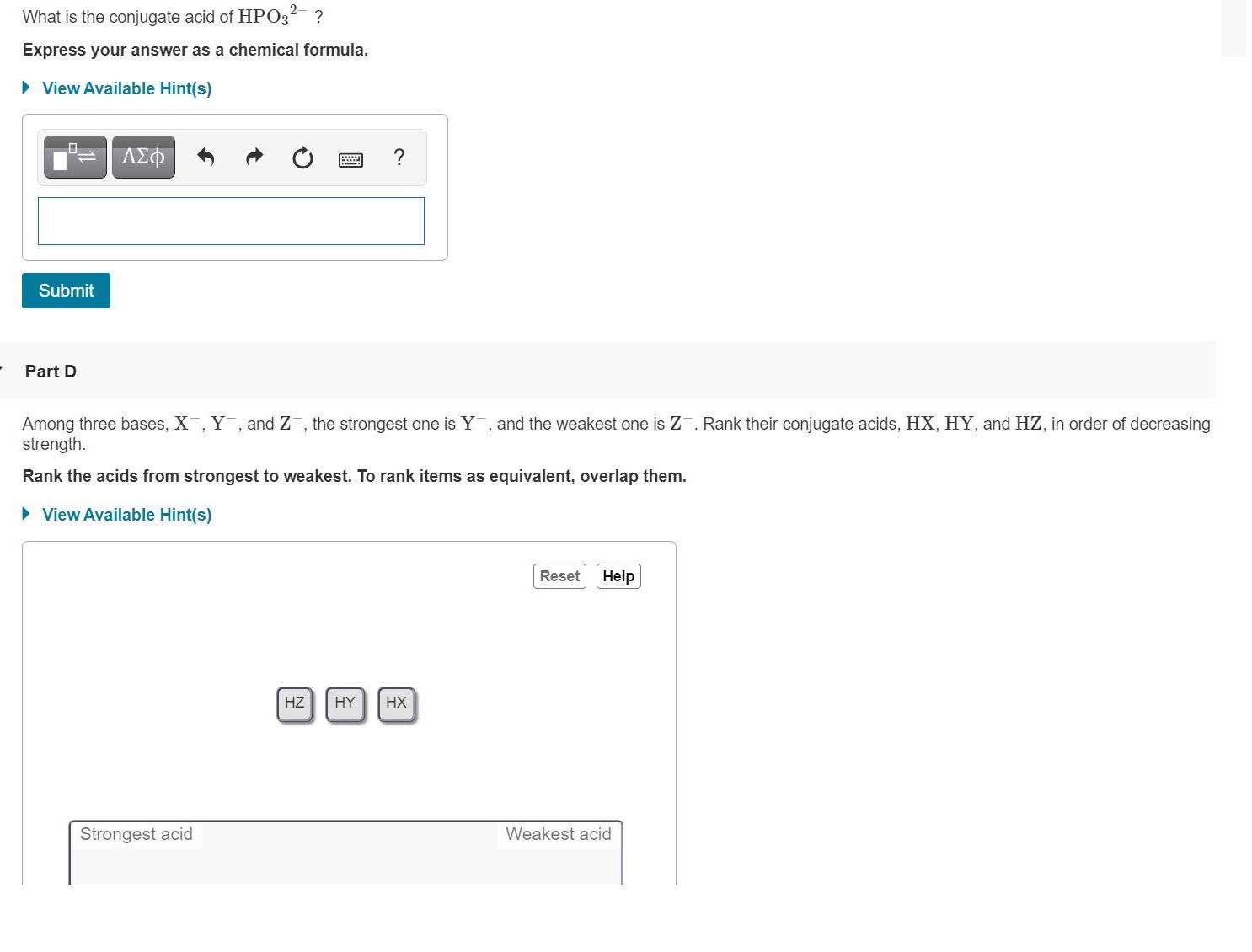
2- What is the conjugate acid of HPO3- ? Express your answer as a chemical formula. View Available Hint(s) B ? Part D Among three bases, X, Y, and Z, the strongest one is Y, and the weakest one is Z. Rank their conjugate acids, HX, HY, and HZ, in order of decreasing strength. Rank the acids from strongest to weakest. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help HZ HY HX Strongest acid Submit | Weakest acid
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Taxation For Decision Makers 2014
Authors: Shirley Dennis Escoffier, Karen Fortin
6th Edition
978-1118654545
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



