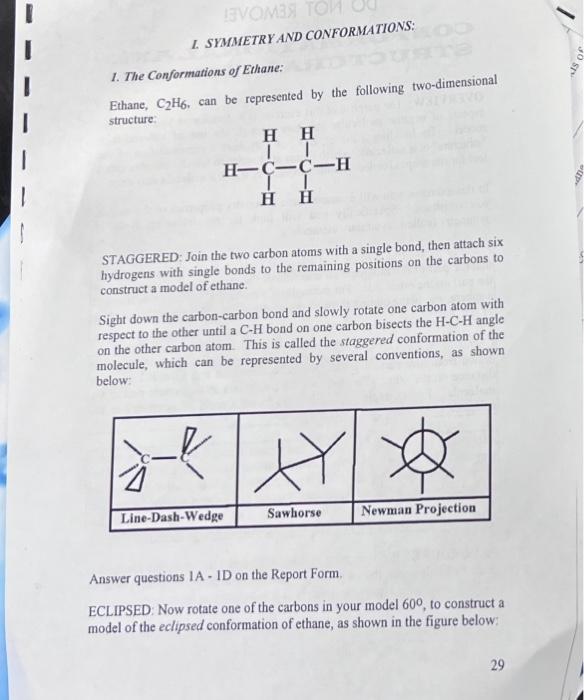
I. SYMMETRY AND CONFORMATIONS: ETHANE - staggered conformation: 1A Does the staggered conformation of ethane have any symmetry planes? If so, describe the location of one of them. 1B Describe the location of a three-fold rotational axis of symmetry that the staggered conformation has. (Use terms like "along the C-C bond," or "perpendicular to the C-C bond," etc.) 1C Describe the location of a two-fold rotational axis of symmetry that the staggered conformation has. 1D Does this conformation of ethane contain a center of symmetry? If so, describe its location. - FOR LAB USE DO NOT REMOVE! OVERVIEW: The purpose of this exercise is to familiarize you with some aspects of organic molecular structure through the use of ball-and-stick models. By building a model, one not only sees a representation of the "structure" of a molecule - which atoms are bonded to which - but also gets a feel for the shapes (called "conformations") in which a molecule may exist. In this exercise, you will examine some symmetry properties of the models you construct. Specifically you will look for the following types of symmetry (called "symmetry elements"). Plane of Symmetry: a plane that passes through a molecule in such a way that the part of the molecule on one side of the plane is the mirror image of the part on the other side. For example, if a molecule had the exact shape of a sphere, any plane cutting through the center of the sphere would be a plane of symmetry. Center of Symmetry: a point within a molecule such that a straight line drawn from any part of the molecule to this point and extended an equal distance on the other side of this point encounters an equivalent part of the molecule. For example an H2 molecule has a point of symmetry at the midpoint of the HH bond. Axis of Symmetry: an axis or line that passes through a molecule in such a way that rotation of the entire molecule about the axis by a certain number of degrees brings the molecule to a position indistinguishable from the original. For example, an equilateral triangle has a threefold axis of rotation, perpendicular to the plane of the triangle. This axis is threefold because rotation of the triangle by 1200(3600/3) retums it to its original position. PROCEDURE: Work in pairs. Using your model kit, assemble the models described below and then answer the questions on the report form (pages 34 - 42). Remove the report form and turn it in before you leave. 1. The Conformations of Ethane: Ethane, C2H6, can be represented by the following two-dimensional structure: STAGGERED: Join the two carbon atoms with a single bond, then attach six hydrogens with single bonds to the remaining positions on the carbons to construct a model of ethane. Sight down the carbon-carbon bond and slowly rotate one carbon atom with respect to the other until a CH bond on one carbon bisects the HCH angle on the other carbon atom. This is called the staggered conformation of the molecule, which can be represented by several conventions, as shown below: Answer questions 1A - ID on the Report Form. ECLIPSED: Now rotate one of the carbons in your model 60, to construct a model of the eclipsed conformation of ethane, as shown in the figure below