I tried to answer some questions on my own but i am failing to continue kindly assist with number 4 and 5 as well.
the two below are answers that were obtained when solving number 2


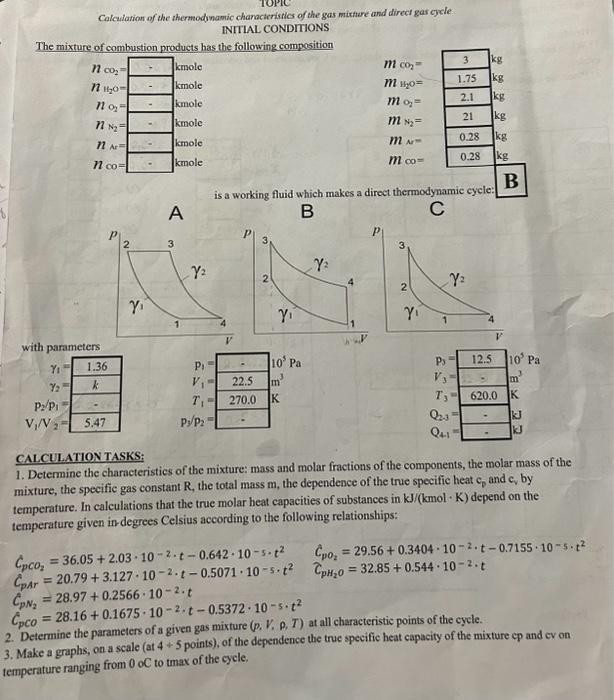
Calculation of the thermodinamic chanacteristios of the gas mixture and direct gas cyele INITIAL CONDITIONS The mixture of combustion products has the following composition with parameters is a working fluid which makes a direct thermodynamic cycle: A B C p) CALCHLATION TASKS: 1. Determine the characteristics of the mixture: mass and molar fractions of the components, the molar mass of the temperature. In ealculations that the true molar heat capacities of substances in kJ/(kmolK) depend on the temperature given in-degrees Celsius according to the following relationships: CpCO2=36.05+2.03102t0.642105t2CpO2=29.56+0.3404102t0.7155105t2CpAr=20.79+3.127102t0.5071105t2CpH20=32.85+0.544102tCpN2=28.97+0.2566102.tCpCO2=28.16+0.1675102.t0.5372105t2Determinetheparametersofagivengasmixture(p,V,,.T)atallcharacteristicpointsofthecycle. 2. Determine the parameters of a given gas mixture (p,V,p,T) at all characteristic points of the cycle. 3. Make a graphs, on a scale (at 4+5 points), of the dependence the true specific heat capacity of the mixture cp and cv on emperature ranging from 0 oC to tmax of the cycle. 4. For process 2-3, determine the values of Q,U,H, taking into account the dependence of heat capacity on temperature. Compare the obtained values with the corresponding process values 2-3 calculated at a constant heat capacity corresponding to the temperature 12. 5. For each process, calculate the values of the work L, the amount of heat Q, the change in internal energy U, the enthalpy H, and the heat distribution coeflicient 6. Determine for the cycle the number of summed (Q1) and allocated (Q2) and algebraic sums U,H,S,L,Q 7. Draw the cycle in the coordinates pV and T-S at the true seale, at characteristic points. 8. For each process, give a diagram of the energy distribution and sbow schematically the areas in pV and TS coordinates corresponding to U,H4,Q 9. For a polytropic process that does not coincide with special cases, show schematieally the areas in pV and T-S coordinates corresponding to U,H,1,Q 10. Derermine the efficiency of this cycle and compare it with the efficiency of the Carnot cycle operating in the sime temperature range. 11. Formulate recommendations to improve the efficiency of the cycle. Attention: 1) When calculating the cyele take into account that cp,cpuk are constants and calculate them at minimum temperatureof the cycle. 2) Place the calculation results in the tables: 2. Paraculers of given gos wixture. (1-2) Actigibatic coupteorion s p8=CP1V1