Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I typed in rate =0.003[A]^0[B]^1/2 I'm not sure where I'm wrong on my math Use the initial rates method with the given data to determine
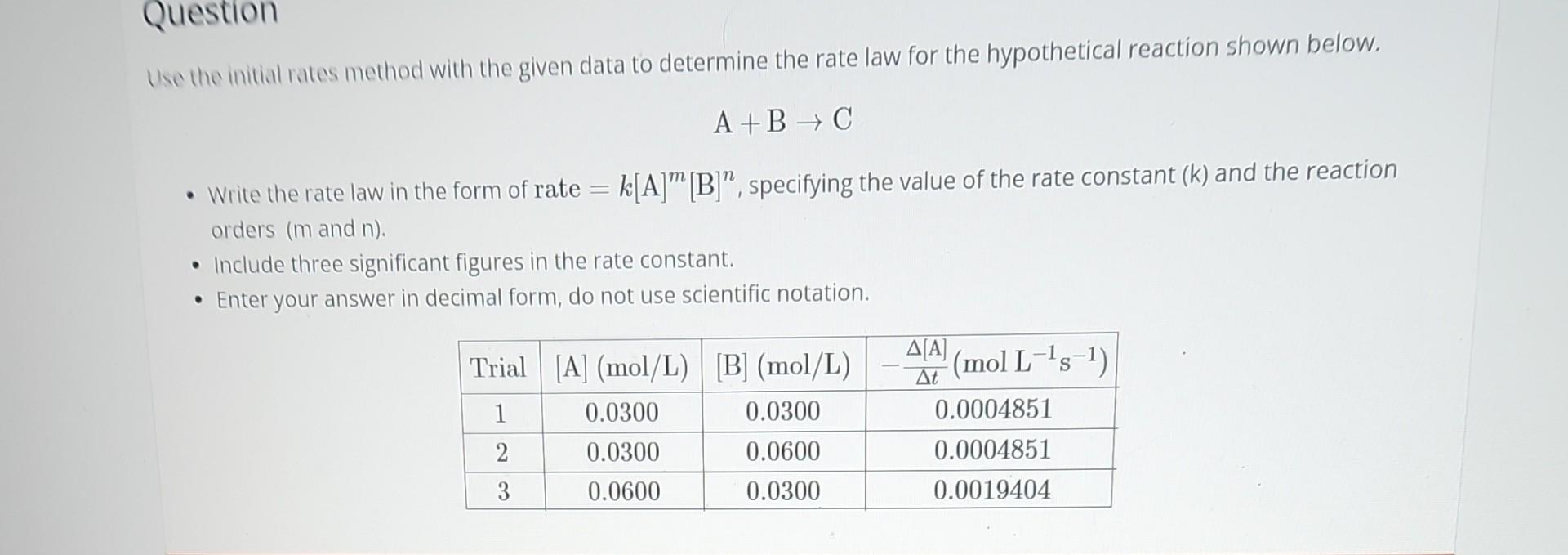
I typed in rate =0.003[A]^0[B]^1/2 I'm not sure where I'm wrong on my math
Use the initial rates method with the given data to determine the rate law for the hypothetical reaction shown below. A+BC - Write the rate law in the form of rate =k[A]m[B]n, specifying the value of the rate constant ( (k) and the reaction orders ( m and n ). - Include three significant figures in the rate constant. - Enter your answer in decimal form, do not use scientific notationStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


