Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I WANT TO ASK THE 2ND QUESTION !! A hydrogel is composed of crosslinked polymer chains, which are highly water absorbent. The gels are mostly
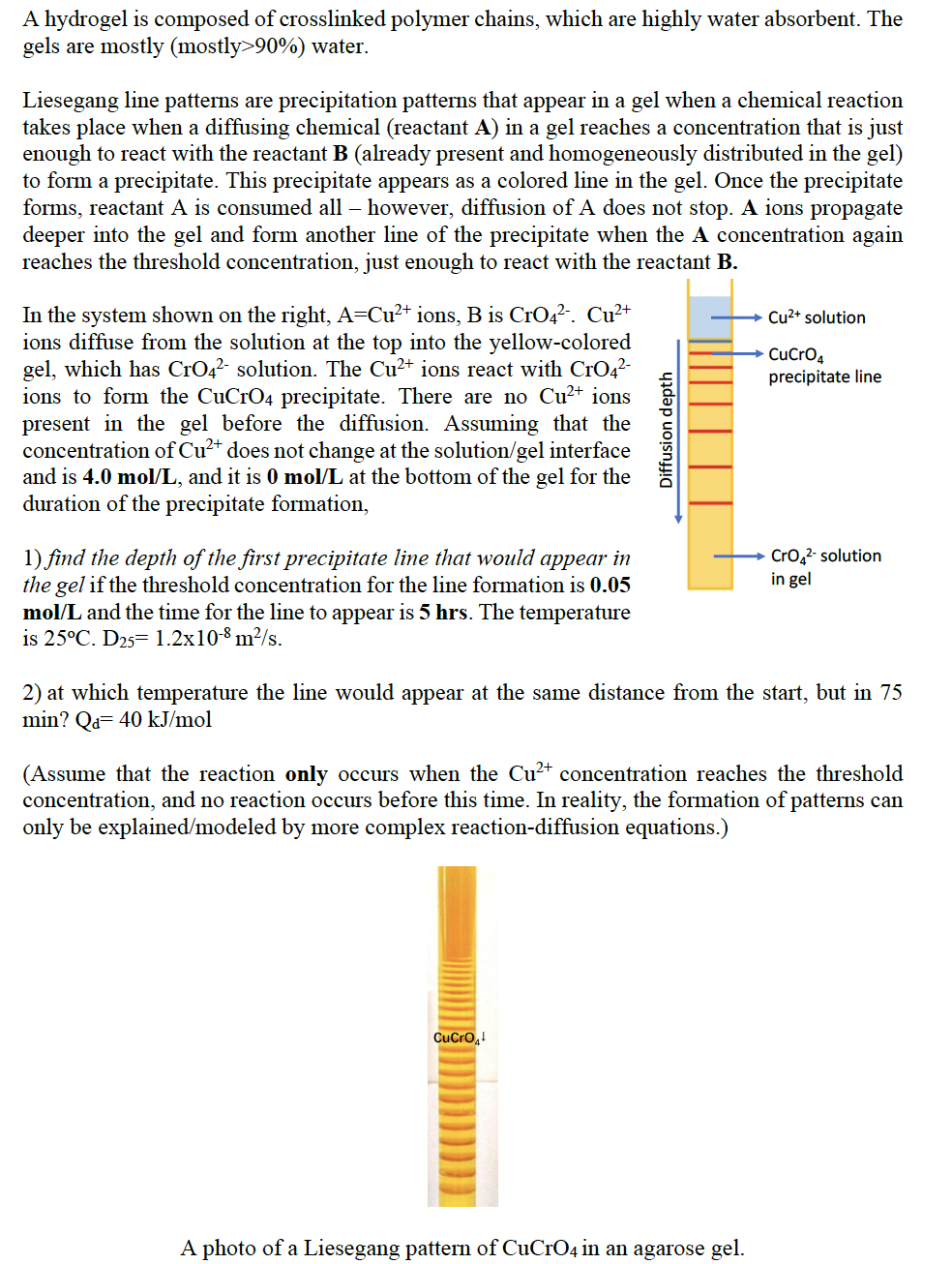
I WANT TO ASK THE 2ND QUESTION !!
A hydrogel is composed of crosslinked polymer chains, which are highly water absorbent. The gels are mostly (mostly >90%) water. Liesegang line patterns are precipitation patterns that appear in a gel when a chemical reaction takes place when a diffusing chemical (reactant A ) in a gel reaches a concentration that is just enough to react with the reactant B (already present and homogeneously distributed in the gel) to form a precipitate. This precipitate appears as a colored line in the gel. Once the precipitate forms, reactant A is consumed all - however, diffusion of A does not stop. A ions propagate deeper into the gel and form another line of the precipitate when the A concentration again reaches the threshold concentration, just enough to react with the reactant B. In the system shown on the right, A=Cu2+ ions, B is CrO42.Cu2+ ions diffuse from the solution at the top into the yellow-colored gel, which has CrO42 solution. The Cu2+ ions react with CrO42 ions to form the CuCrO4 precipitate. There are no Cu2+ ions present in the gel before the diffusion. Assuming that the concentration of Cu2+ does not change at the solution/gel interface and is 4.0mol/L, and it is 0mol/L at the bottom of the gel for the duration of the precipitate formation, 1) find the depth of the first precipitate line that would appear in the gel if the threshold concentration for the line formation is 0.05 mol/L and the time for the line to appear is 5hrs. The temperature is 25C.D25=1.2108m2/s. 2) at which temperature the line would appear at the same distance from the start, but in 75 min?Qd=40kJ/mol (Assume that the reaction only occurs when the Cu2+ concentration reaches the threshold concentration, and no reaction occurs before this time. In reality, the formation of patterns can only be explained/modeled by more complex reaction-diffusion equations.) A photo of a Liesegang pattern of CuCrO4 in an agarose gelStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


