Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I will like the correct answer kl of heat is required to convert 32.575 g of liquid 2-propanol, C3H7OH, at -0.1 C to gaseous 2-propanol
I will like the correct answer 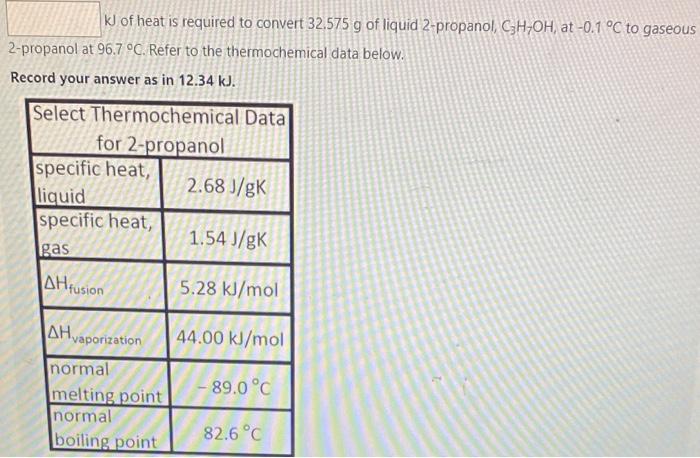
kl of heat is required to convert 32.575 g of liquid 2-propanol, C3H7OH, at -0.1 C to gaseous 2-propanol at 96.7 C. Refer to the thermochemical data below. Record your answer as in 12.34 kJ. Select Thermochemical Data for 2-propanol specific heat, 2.68 J/gK liquid specific heat, 1.54 J/gK gas 5.28 kJ/mol Alfusion 44.00 kJ/mol , vaporization normal melting point normal boiling point 89.0C 82.6 C 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


