Question
If a solution containing 63.00 g of mercury(1I) nitrate is allowed to react completely with a solution containing 17.796 g of sodium dichromate, how
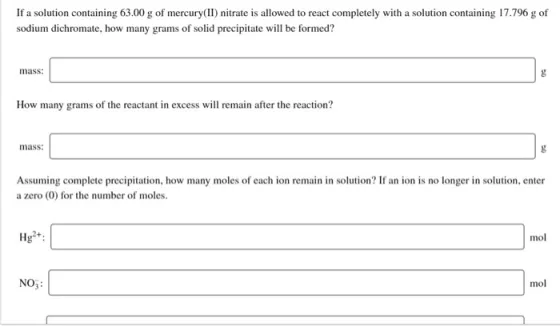
If a solution containing 63.00 g of mercury(1I) nitrate is allowed to react completely with a solution containing 17.796 g of sodium dichromate, how many grams of solid precipitate will be formed? mass: How many grams of the reactant in excess will remain after the reaction? mass: Assuming complete precipitation, how many moles of each ion remain in solution? If an ion is no longer in solution, enter a zero (0) for the number of moles. Hy+: mol NO,: mol
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
The reaction of mercury II nitrate with sodium dichromate can be written as Note that NaNO3 is soluble in aqueous solution but HgCr2O7 is not soluble ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



