Question
If an objecct is heated, the temperature of the body will increase. The energy (Q) associated with a change in temperature (deltaT) is a function
If an objecct is heated, the temperature of the body will increase. The energy (Q) associated with a change in temperature (deltaT) is a function of the mass of the object (m) and the specific heat (Cp). In an experiment, heat is applied to the end of an object, and the temperature change at the other end of the object is recorded. This leads to the theoretical relationship shown. An unknown material is tessted in the lab, yielding the following results: 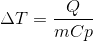
| Heat Applied (Q) [J] | 12 | 17 | 25 | 40 | 50 | 58 |
| Temp Change (deltaT) [K] | 1.50 | 2.00 | 3.25 | 5.00 | 6.25 | 7.00 |
USING MATLAB:
a) Create a proper plot of the experimental temperature change (deltaT, ordinate) versus the heat applied (Q, abscissa).
b) Use polyfit to determine a linear relationship for the data set and graph the resulting trendline along with the experimental data.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


