Question
Table 2: Volume (mL) and temperature changes (C) of distilled water used to calibrate the calorimeter. Trial Volume cold water (+ Volume hot water
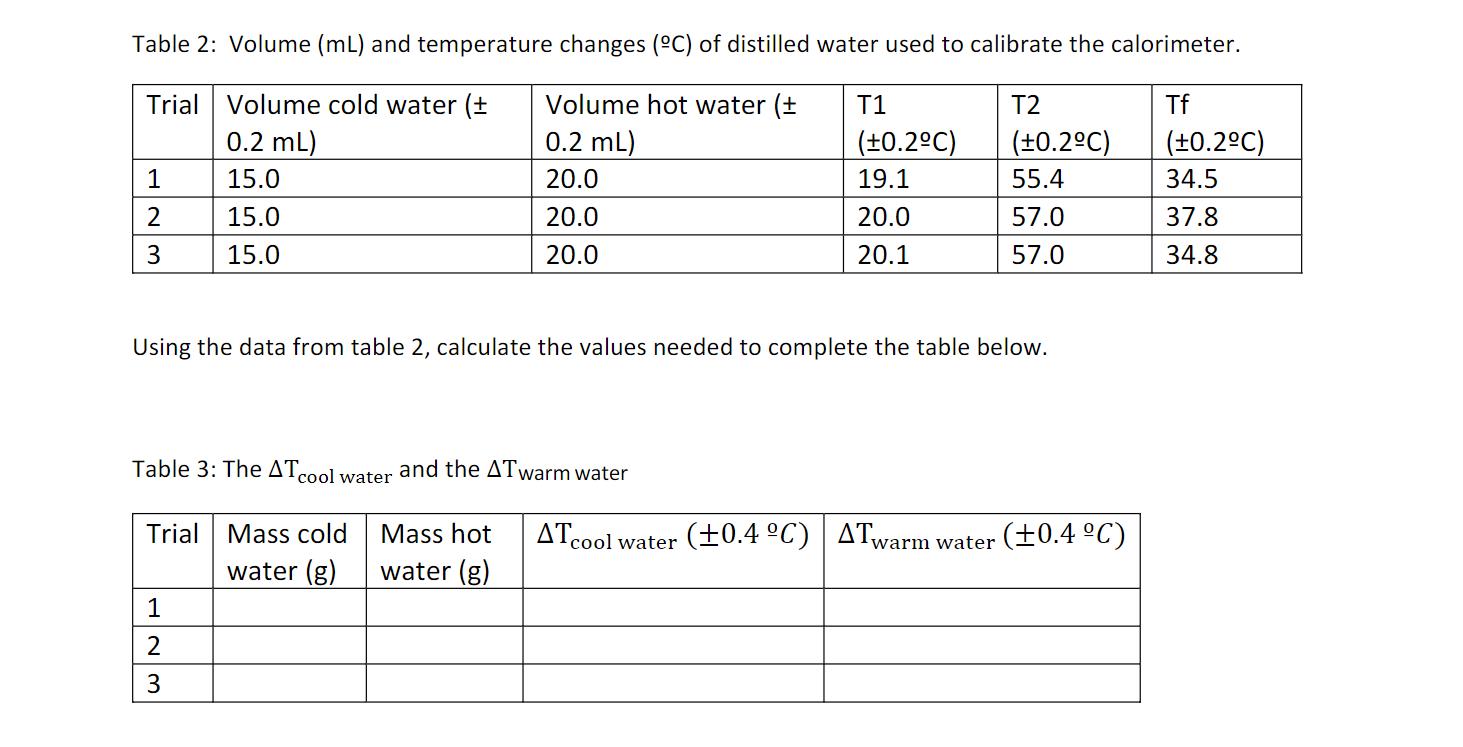
Table 2: Volume (mL) and temperature changes (C) of distilled water used to calibrate the calorimeter. Trial Volume cold water (+ Volume hot water (+ 0.2 mL) 0.2 mL) 15.0 20.0 15.0 20.0 15.0 20.0 1 2 3 Table 3: The ATcool water and the ATwarm water Trial Mass cold Mass hot water (g) water (g) Using the data from table 2, calculate the values needed to complete the table below. 1 2 W|N T1 (0.2C) 19.1 20.0 20.1 3 T2 (0.2C) 55.4 57.0 57.0 ATcool water (+0.4 C) ATwarm water (0.4 C) Tf (0.2C) 34.5 37.8 34.8
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
T1 is cold T2 is Hot Table 3 The A Tool water and We know that density and density of pra...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
System Dynamics
Authors: William Palm III
3rd edition
73398063, 978-0073398068
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



