Question
If the Kea for the forward reaction is greater than 1.0 , the concentration of the reactants will be greater than the concentration of products
If the Kea for the forward reaction is greater than 1.0 , the concentration of the reactants will be greater than the concentration of products at equilibrium.\ The more negative the value for
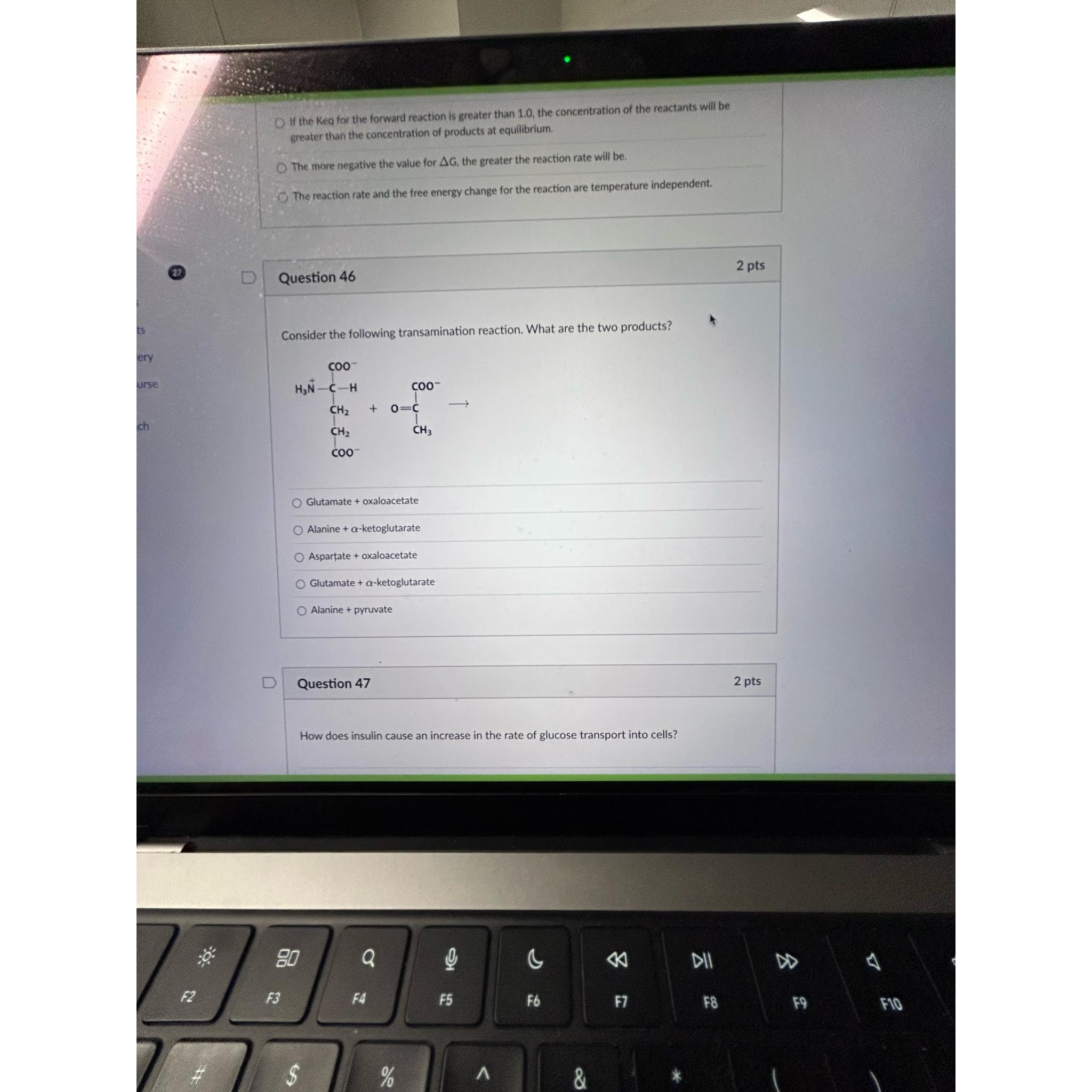
\\\\Delta G, the greater the reaction rate will be.\ The reaction rate and the free energy change for the reaction are temperature independent.\ Question 46\ 2 pts\ Consider the following transamination reaction. What are the two products?\ Glutamate + oxaloacetate\ Alanine
+\\\\alpha -ketoglutarate\ Aspartate + oxaloacetate\ Glutamate
+\\\\alpha -ketoglutarate\ Alanine + pyruvate\ Question 47\ 2 pts\ How does insulin cause an increase in the rate of glucose transport into cells?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


