Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If wi= 37.53 g the bottle is empty W2 = 136.62 g The bottle contains water W3 = 119.45g - The bottle contains light gas

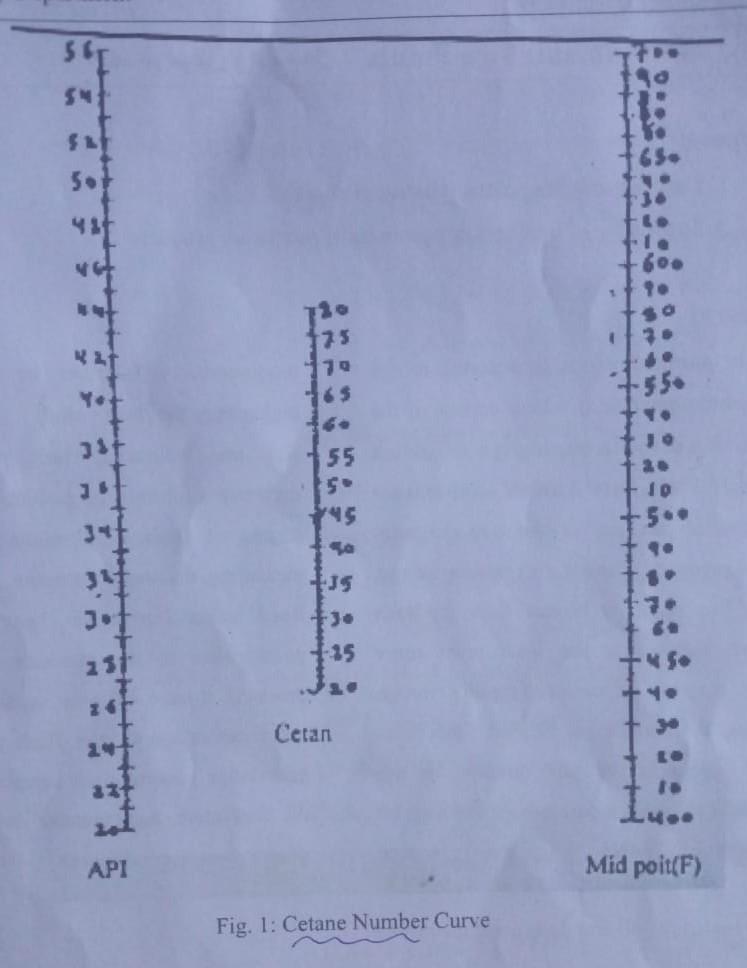
If wi= 37.53 g the bottle is empty W2 = 136.62 g The bottle contains water W3 = 119.45g - The bottle contains light gas oil Tmix = 57 Tseporation = 540 Calculate I aniline Point. 121 Die Sel Index. 13 Cetane Number if Mid Point 550f SAT So 5 3 SR 65. 5 3. to 1 600 SO 3 Lt 175 79 165 Hot 55. ist 55 5 45 ID 56. 34 s. JS tuo Jo 231 125 - S -4. 11 Cetan E 33+ fo API Mid poit(F) Fig. 1: Cetane Number Curve If wi= 37.53 g the bottle is empty W2 = 136.62 g The bottle contains water W3 = 119.45g - The bottle contains light gas oil Tmix = 57 Tseporation = 540 Calculate I aniline Point. 121 Die Sel Index. 13 Cetane Number if Mid Point 550f SAT So 5 3 SR 65. 5 3. to 1 600 SO 3 Lt 175 79 165 Hot 55. ist 55 5 45 ID 56. 34 s. JS tuo Jo 231 125 - S -4. 11 Cetan E 33+ fo API Mid poit(F) Fig. 1: Cetane Number Curve
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


