Answered step by step
Verified Expert Solution
Question
1 Approved Answer
if you could write out the question neatly instead of typing that would be apprciated... Will leave a rating - Thank you QUESTION 2 The
if you could write out the question neatly instead of typing that would be apprciated... Will leave a rating
Thank you
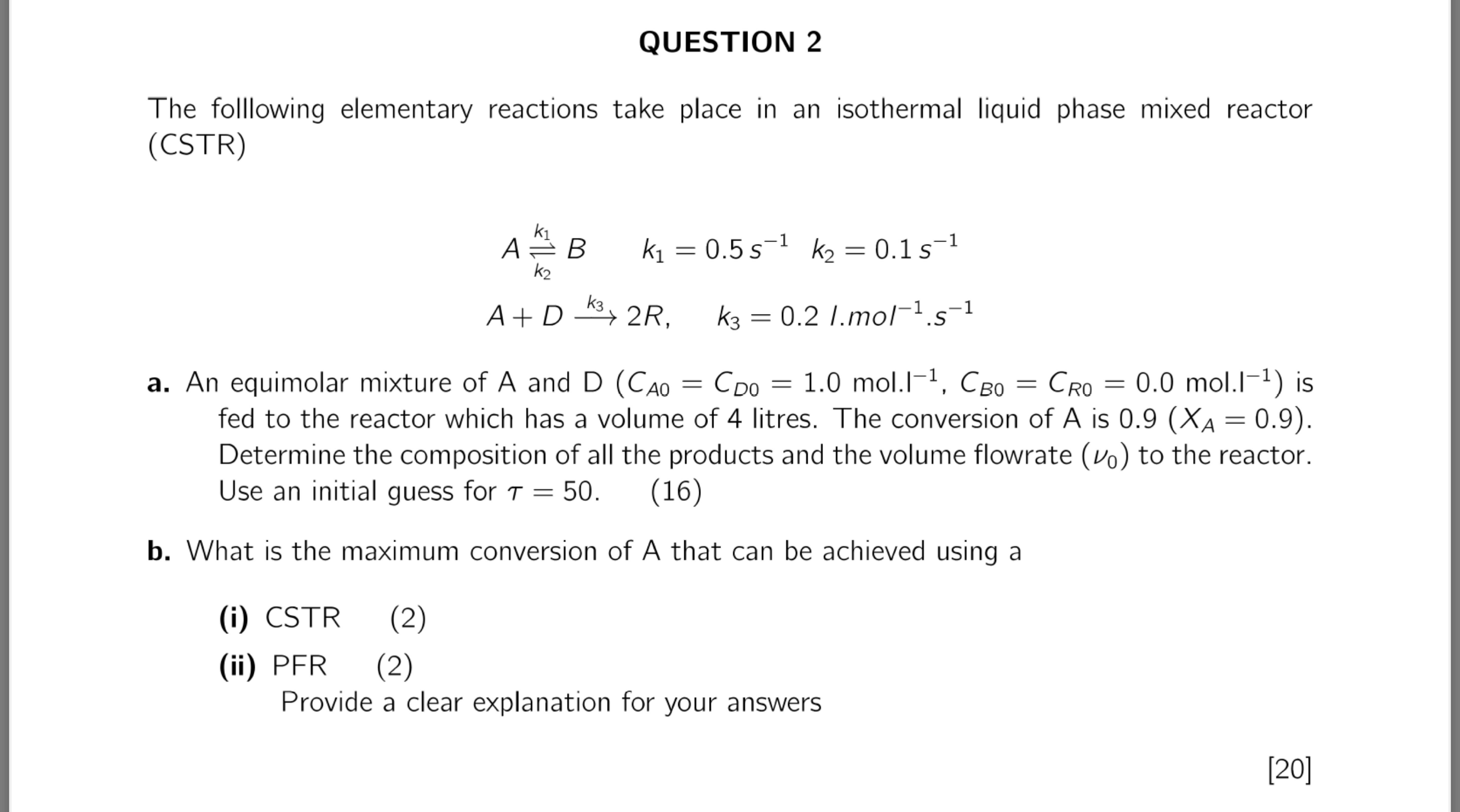
QUESTION
The following elementary reactions take place in an isothermal liquid phase mixed reactor
CSTR
a An equimolar mixture of A and mol. :mol. is
fed to the reactor which has a volume of litres. The conversion of is
Determine the composition of all the products and the volume flowrate to the reactor.
Use an initial guess for
b What is the maximum conversion of A that can be achieved using a
i CSTR
ii PFR
Provide a clear explanation for your answers
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


