Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If you make up a solution of 50mL of 0.1M TRIS in the acid form, what will be the pH ?] K_(a)=((H^(+)3[s^(-)]])/([BH]) TRIS Ka_(a)=8.32*10^(-9) {
If you make up a solution of
50mLof
0.1MTRIS in the acid form, what will be the
pH?]\
K_(a)=((H^(+)3[s^(-)]])/([BH]) TRIS Ka_(a)=8.32*10^(-9)\ {(
:8.32\\\\times 10^(-9)=([H^(-1)][B^(-)))/(8.1),PH=-log(2.88).10^(-5))}
\ [11^(+)]=\\\\sqrt(8.32\\\\times 10^(-4.0.1)),PH=4.54\ =2.84\\\\times 10^(-5)\ If you add
2mLof
1MNaOHto the solution in step 5 , what will be the
pH?
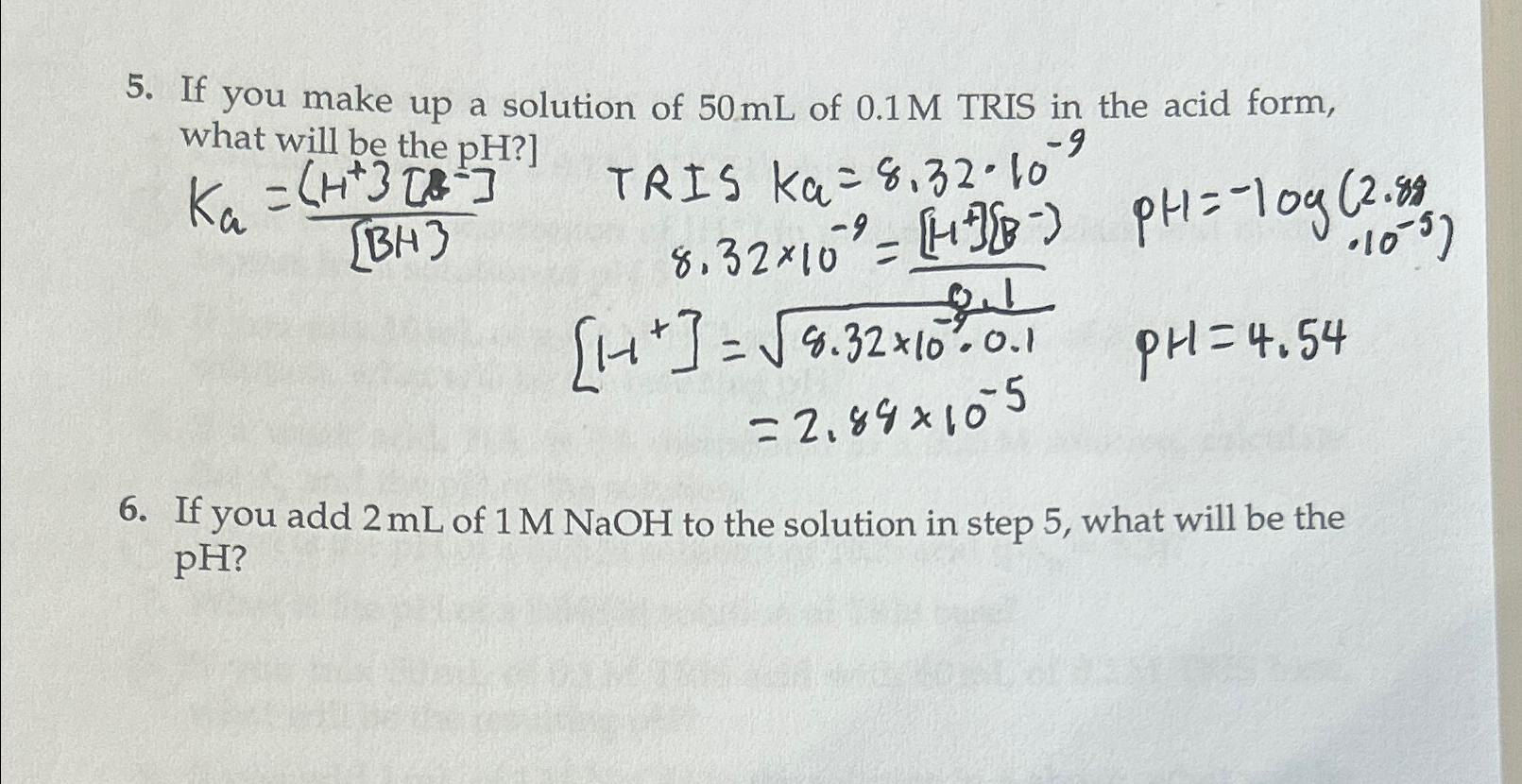
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


