Question
ily.do?locator assignment-take&takeAssignmentSessionl.ocator-assignment-take sAssign C Chegg Revie Tepia Retarences Use the References arcen impertant values if eeded fer thi quntion. The equilibrium constant, K. for
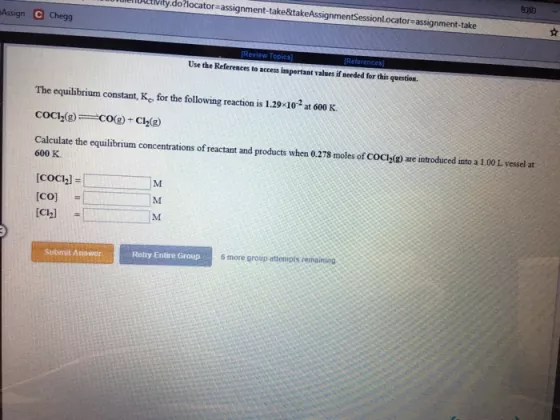
ily.do?locator assignment-take&takeAssignmentSessionl.ocator-assignment-take sAssign C Chegg Revie Tepia Retarences Use the References arcen impertant values if eeded fer thi quntion. The equilibrium constant, K. for the following reaction is 1.29-10 at 600K. COC(e) CO(g)- Cl2) Calculate the equilibrium concentrations of reactant and products when 0.278 moles of COC(g are introduced unto a 100L vessel at 600 K [COCI,] = M (CO) M %3D [Ch] M Sabmut Anr Rely Entire Group Smore group atenipls remainseg
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Solution co9 cog 129162 2 ak bese K 07mole...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



