Answered step by step
Verified Expert Solution
Question
1 Approved Answer
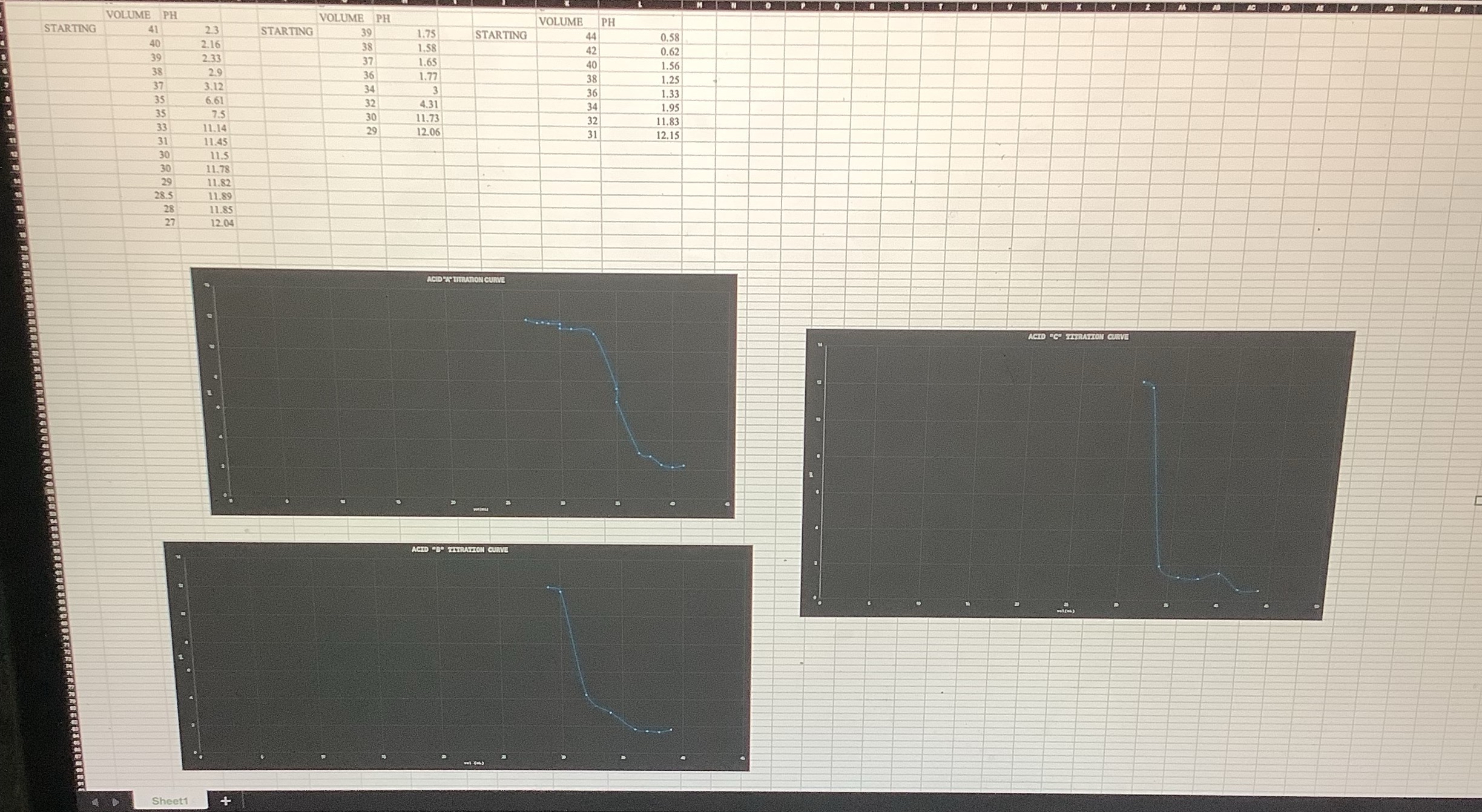
im doing a acids and bases post lab for chem 2 and need help ASAP!!! for the problems below ( USE THE DATA IN THE
im doing a acids and bases post lab for chem and need help ASAP!!! for the problems below USE THE DATA IN THE PICTURE TO ANSWER THE QUESTIONS BELOW
For each acid below, indicate the unknown identity A B or C based on graphs ac which acid best corresponds to it
HCl
HPO
HCO
WHAT IS THE MOLARITY DETERMINED FOR YOUR SOLUTION OF HCl
WHAT IS THE MOLARITY DETERMINED FOR YOUR SOLUTION OF HPO
WHAT IS THE MOLARITY DETERMINED FOR YOUR SOLUTION OF HCO
Please explain how half equivalence points were used in the identification of the acids you titrated. Be sure to use the values you determined for the acids titrated in your explanation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


