Question
Determine the molar enthalpy of combustion of hydrogen sulfide [HS(g)], given the following thermochemical equations. [5 marks] 2 HS(g) +30(g) t 2 SO2(g) +2
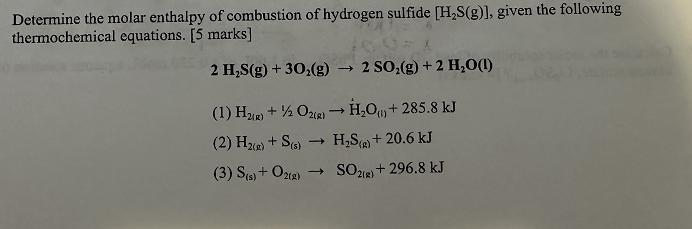
Determine the molar enthalpy of combustion of hydrogen sulfide [HS(g)], given the following thermochemical equations. [5 marks] 2 HS(g) +30(g) t 2 SO2(g) +2 HO(1) (1) H2) + O2(g) HO() + 285.8 kJ (2) H2(g) + S(5) -> HS (g) + 20.6 kJ (3) S(s) + O2(g) -> SO2(g) + 296.8 kJ
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Solution To determine the molar enthalpy of combustion of hydrogen sulfide H2S we can use the giv...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Java Programming And Data Structures Comprehensive Version
Authors: Y. Daniel Liang
12th Edition
0136520235, 978-0136520238
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



