Answered step by step
Verified Expert Solution
Question
1 Approved Answer
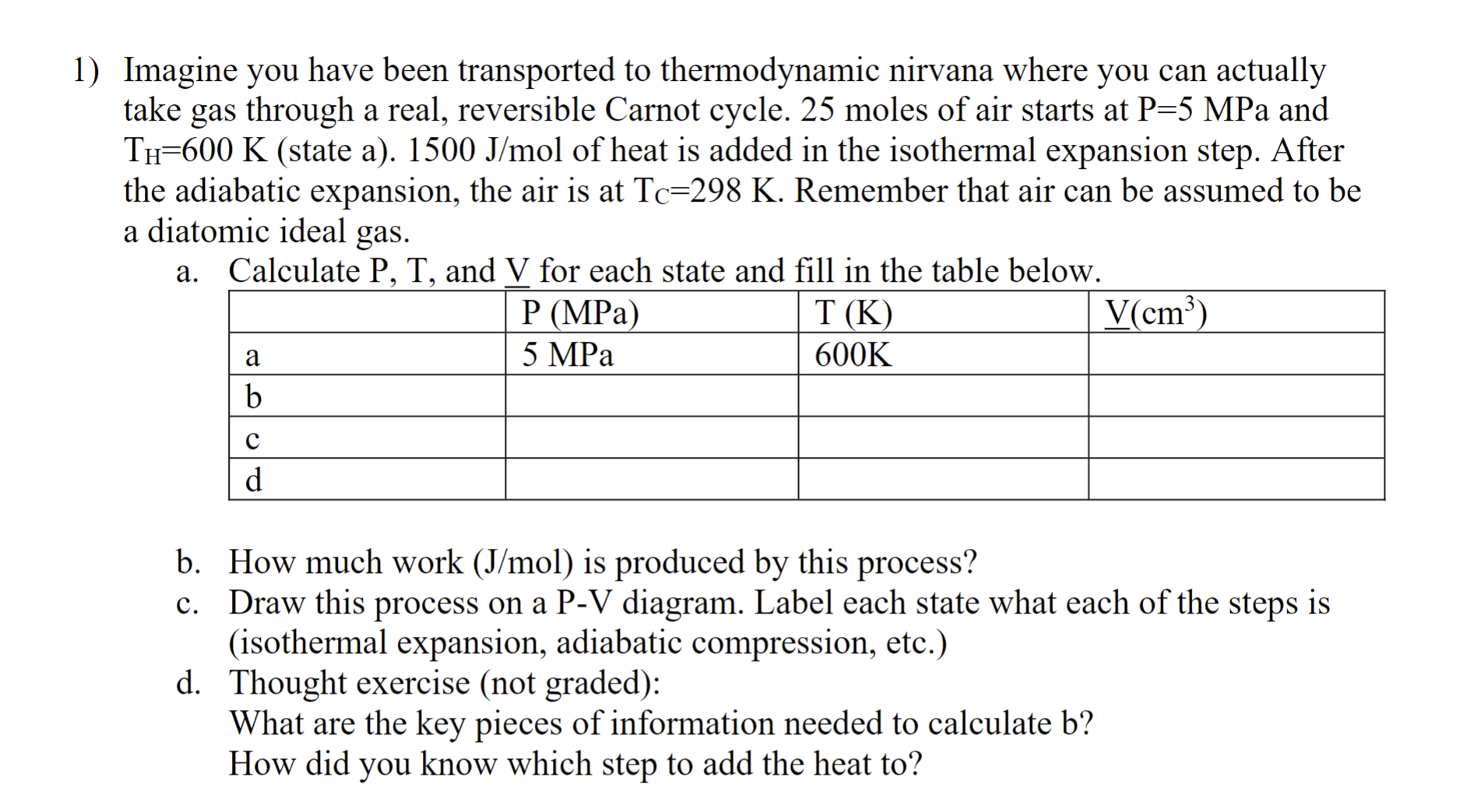
Imagine you have been transported to thermodynamic nirvana where you can actually take gas through a real, reversible Carnot cycle. 2 5 moles of air
Imagine you have been transported to thermodynamic nirvana where you can actually
take gas through a real, reversible Carnot cycle. moles of air starts at MPa and
state a of heat is added in the isothermal expansion step. After
the adiabatic expansion, the air is at Remember that air can be assumed to be
a diatomic ideal gas.
a Calculate and for each state and fill in the table below.
b How much work
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


