Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Implement a molecular dynamics simulation for N = 4 9 particles enclosed in a square box of 8 A by 8 A , the walls
Implement a molecular dynamics simulation for N particles enclosed in a square box of A by A the walls of the box have the condition of performing elastic collisions. The particles are positioned so that they are separated by angstrom between them and with a uniform velocity distribution, which interact with a LennardJones type potential. Carry out the simulation forepsilon and sigma at times long enough to observe the changes in the distribution of speeds and temperature, as well as the changes in its potential energy, kinetic energy and its total energy, that is obtain the graphs of KE vs t PE vs t Etotal vs t and T vs t
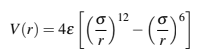
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


