Answered step by step
Verified Expert Solution
Question
1 Approved Answer
important: I don't understand the step by step to clear the constant k1, nor do I understand why the unit of k1 is gmol/(L.h.atm). Why
important: I don't understand the step by step to clear the constant k1, nor do I understand why the unit of k1 is gmol/(L.h.atm). Why atmosphere?
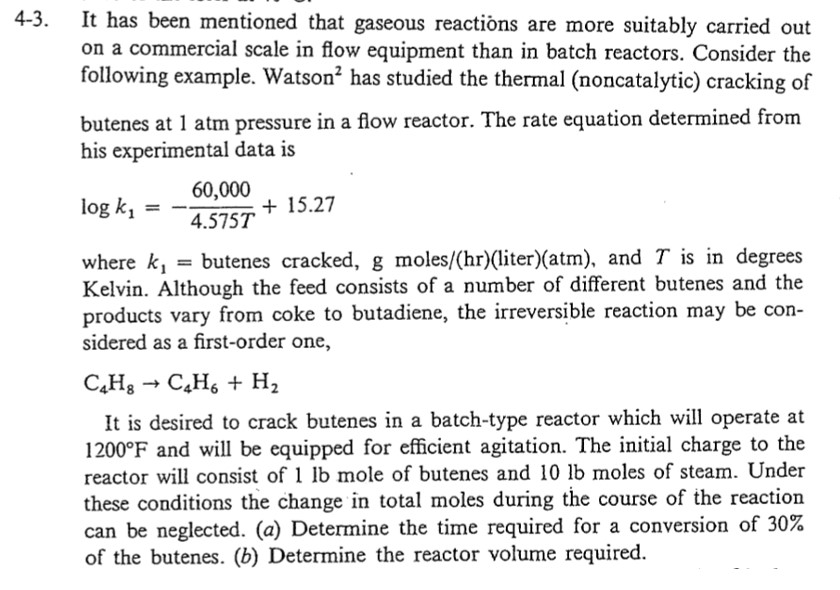 It has been mentioned that gaseous reactions are more suitably carried out on a commercial scale in flow equipment than in batch reactors. Consider the following example. Watson 2 has studied the thermal (noncatalytic) cracking of butenes at 1atm pressure in a flow reactor. The rate equation determined from his experimental data is logk1=4.575T60,000+15.27 where k1= butenes cracked, g moles/(hr)(liter)(atm), and T is in degrees Kelvin. Although the feed consists of a number of different butenes and the products vary from coke to butadiene, the irreversible reaction may be considered as a first-order one, C4H8C4H6+H2 It is desired to crack butenes in a batch-type reactor which will operate at 1200F and will be equipped for efficient agitation. The initial charge to the reactor will consist of 1lb mole of butenes and 10lb moles of steam. Under these conditions the change in total moles during the course of the reaction can be neglected. (a) Determine the time required for a conversion of 30% of the butenes. (b) Determine the reactor volume required
It has been mentioned that gaseous reactions are more suitably carried out on a commercial scale in flow equipment than in batch reactors. Consider the following example. Watson 2 has studied the thermal (noncatalytic) cracking of butenes at 1atm pressure in a flow reactor. The rate equation determined from his experimental data is logk1=4.575T60,000+15.27 where k1= butenes cracked, g moles/(hr)(liter)(atm), and T is in degrees Kelvin. Although the feed consists of a number of different butenes and the products vary from coke to butadiene, the irreversible reaction may be considered as a first-order one, C4H8C4H6+H2 It is desired to crack butenes in a batch-type reactor which will operate at 1200F and will be equipped for efficient agitation. The initial charge to the reactor will consist of 1lb mole of butenes and 10lb moles of steam. Under these conditions the change in total moles during the course of the reaction can be neglected. (a) Determine the time required for a conversion of 30% of the butenes. (b) Determine the reactor volume required Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


