Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Important Notes: - You will need to attend or watch the recorded connect section to fill in all of the information below. - If you

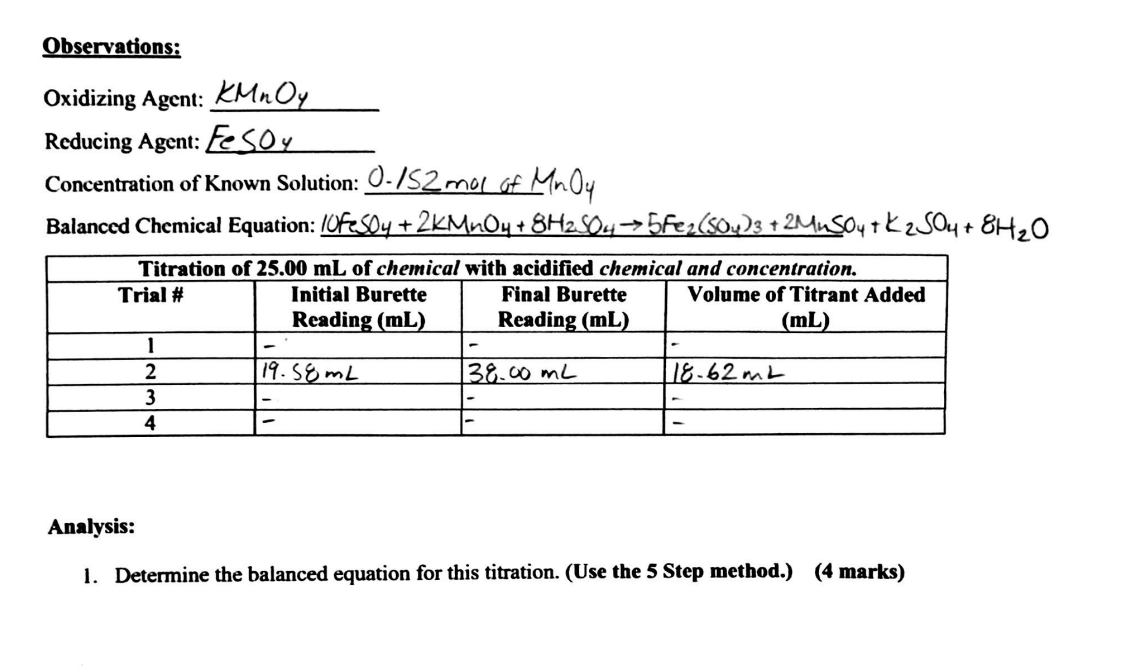
 Important Notes: - You will need to attend or watch the recorded connect section to fill in all of the information below. - If you cannot watch the recording a virtual simulator can be found in the online lesson (U2L3SC). - This is to be submitted with your lab report. Materials: - Burette - 4100mL flask - Stir plate - Stir bar - KMnO4 solution (concentration= 0.152mol/L ) - Fe(SO4) solution (concentration= unknown) - Retort Stand - Sulfuric Acid Procedure: 1. Set up titration equipment 2. Measure out 25.0mL of the unknown Fe(SO4) solution into a 100mL flask. 3. Fill the burette with the KMnO4. 4. Perform four titrations to determine the concentration of the Fe(SO4) solution. Observations: Oxidizing Agent: KMnO4 Reducing Agent: FeSO4 Concentration of Known Solution: 0.1S2malofMnO4 Balanced Chemical Equation: 10FeSO4+2KMnO4+8H2SO45Fe2(SO4)3+2MnSO4+K2SO4+8H2O Analysis: 1. Determine the balanced equation for this titration. (Use the 5 Step method.) (4 marks) 2. What is the concentration of the Fe(SO4) solution? (4 marks) 3. If the actual concentration was 0.572mol/L what is the percent error associated with this titration experiment? (2 marks)
Important Notes: - You will need to attend or watch the recorded connect section to fill in all of the information below. - If you cannot watch the recording a virtual simulator can be found in the online lesson (U2L3SC). - This is to be submitted with your lab report. Materials: - Burette - 4100mL flask - Stir plate - Stir bar - KMnO4 solution (concentration= 0.152mol/L ) - Fe(SO4) solution (concentration= unknown) - Retort Stand - Sulfuric Acid Procedure: 1. Set up titration equipment 2. Measure out 25.0mL of the unknown Fe(SO4) solution into a 100mL flask. 3. Fill the burette with the KMnO4. 4. Perform four titrations to determine the concentration of the Fe(SO4) solution. Observations: Oxidizing Agent: KMnO4 Reducing Agent: FeSO4 Concentration of Known Solution: 0.1S2malofMnO4 Balanced Chemical Equation: 10FeSO4+2KMnO4+8H2SO45Fe2(SO4)3+2MnSO4+K2SO4+8H2O Analysis: 1. Determine the balanced equation for this titration. (Use the 5 Step method.) (4 marks) 2. What is the concentration of the Fe(SO4) solution? (4 marks) 3. If the actual concentration was 0.572mol/L what is the percent error associated with this titration experiment? (2 marks) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


