Question
In a batch reactor, reactant A undergoes a certain reaction to form product B. The concentrations of A and B with respect time are
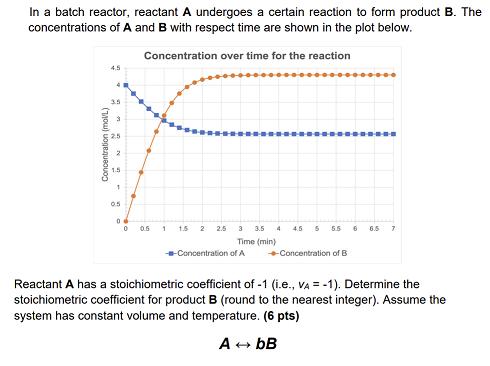
In a batch reactor, reactant A undergoes a certain reaction to form product B. The concentrations of A and B with respect time are shown in the plot below. Concentration over time for the reaction 4,5 35 Concentration (mol/L) 3 0.5 0 0 1 1.5 2 2.5 3 3.5 4 4.5 Time (min) -Concentration of A 5 5.5 6 -Concentration of B 6.5 7 Reactant A has a stoichiometric coefficient of -1 (i.e., VA = -1). Determine the stoichiometric coefficient for product B (round to the nearest integer). Assume the system has constant volume and temperature. (6 pts) A+bB
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



