Question
In a cell, respiration reaction occurs as follows: C6H12O6 + O2 CO2 + H2O O2 diffuses through cell membrane of 10 nm thickness to the
In a cell, respiration reaction occurs as follows: C6H12O6 + O2 CO2 + H2O O2 diffuses through cell membrane of 10 nm thickness to the cell surface and completely converts to CO2 and H2O. Subsequently, CO2 and H2O produced in a cell releases to the cell membrane and eventually returns to the bulk gas. At the bulk gas (37C and 1 atm) mole percents of O2 (A), CO2 (B) and H2O (C) are 0.5, 0.25 and 0.25, respectively.
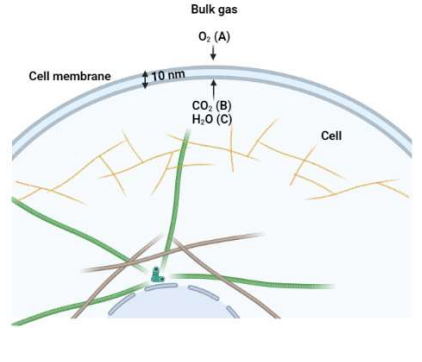
1.1 Calculate the DAB (O2-CO2), DBC (CO2-H2O) and DAC (O2-H2O) at 37C and 1 atm.
1.2 Please balance the reaction equation stoichiometrically, calculate the effective diffusivity and total molar fluxes of O2, CO2 and H2O
Bulk gas Cell membrane +10nm O2(A) Bulk gas Cell membrane +10nm O2(A)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


