Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In a class on head loss of pipes, these data were obtained: roughness = 0.015 mm Reservoir T = 20 C L = 30 cm
In a class on head loss of pipes, these data were obtained: roughness = 0.015 mm Reservoir
T = 20 C L = 30 cm 0.30 m h= 20.7 cm > 0.207 m D = 1 inch 0.0254 m > 0,0254 m Lt= 6.6 cm + 51 cm + 14.4 cm + 5.2 cm + 8.1 cm + 9.8 cm + 10.3 + 6.3 + 9 + 9.7 = Lt = 130.4 cm
For each of the conditions find the estimated distributed head loss. Find the localized head loss, from the values of K, for the singularities. Find the total head loss. Present calculations and results in a report
Complementary data
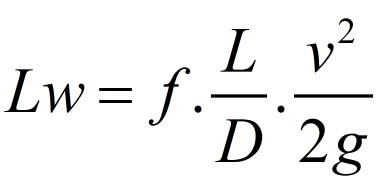
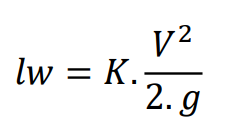
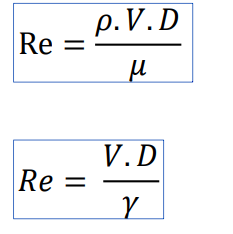
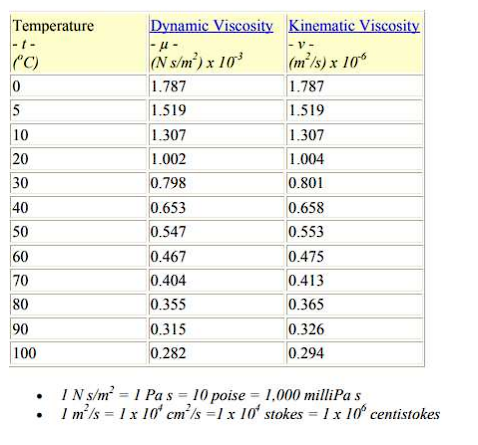
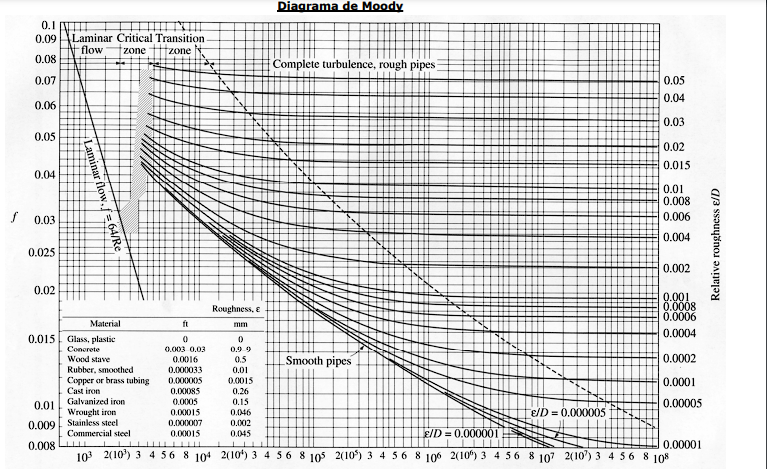
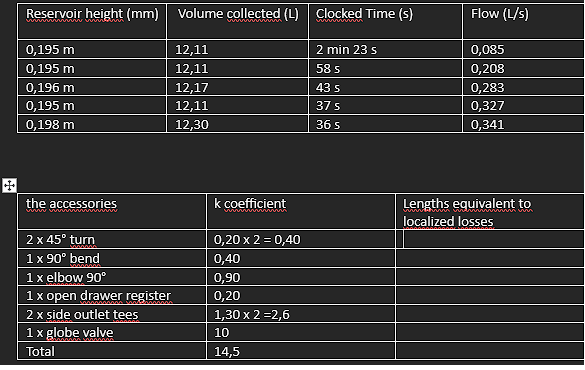
\begin{tabular}{|l|l|l|l|} \hline Reservoir height (mm) & Volume collected (L) & Clocked Time (s) & Flow (L/s) \\ \hline 0,195m & 12,11 & 2min23s & 0,085 \\ \hline 0,195m & 12,11 & 58s & 0,208 \\ \hline 0,196m & 12,17 & 43s & 0,283 \\ \hline 0,195m & 12,11 & 37s & 0,327 \\ \hline 0,198m & 12,30 & 36s & 0,341 \\ \hline \end{tabular} \begin{tabular}{|l|l|l|} \hline the accessories & k coefficient & Lengthsequivalenttolocalizedlosses \\ \hline 245 turn & 0,202=0,40 & \\ \hline 190 bend & 0,40 & \\ \hline 1 elbow 90 & 0,90 & \\ \hline 1 open drawer register & 0,20 & \\ \hline 2 side outlet tees & 1,302=2,6 & \\ \hline 1 globe valve & 10 & \\ \hline Total & 14,5 & \\ \hline \end{tabular} Lw=fDL2gv2 lw=K2gV2 Re=.VD Re=V.D - 1N/m2=1Pas=10 poise =1,000 milliPa s - 1m2/s=1104cm2/s=1104 stokes =1106 centistok Diagrama_de Moody
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


