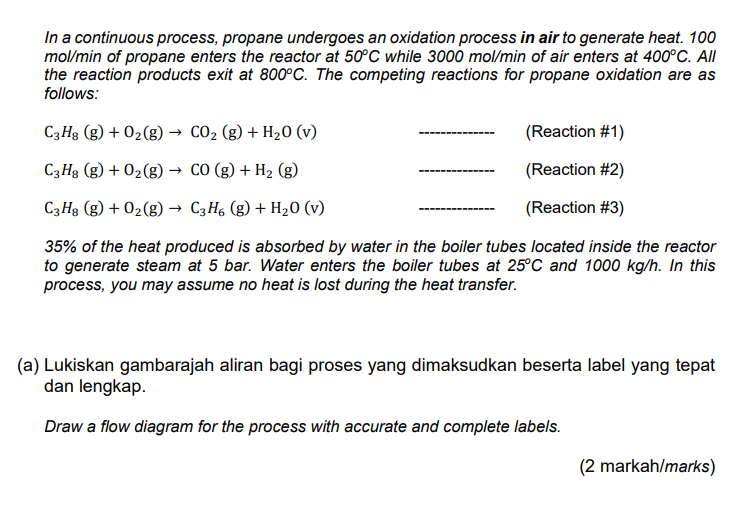
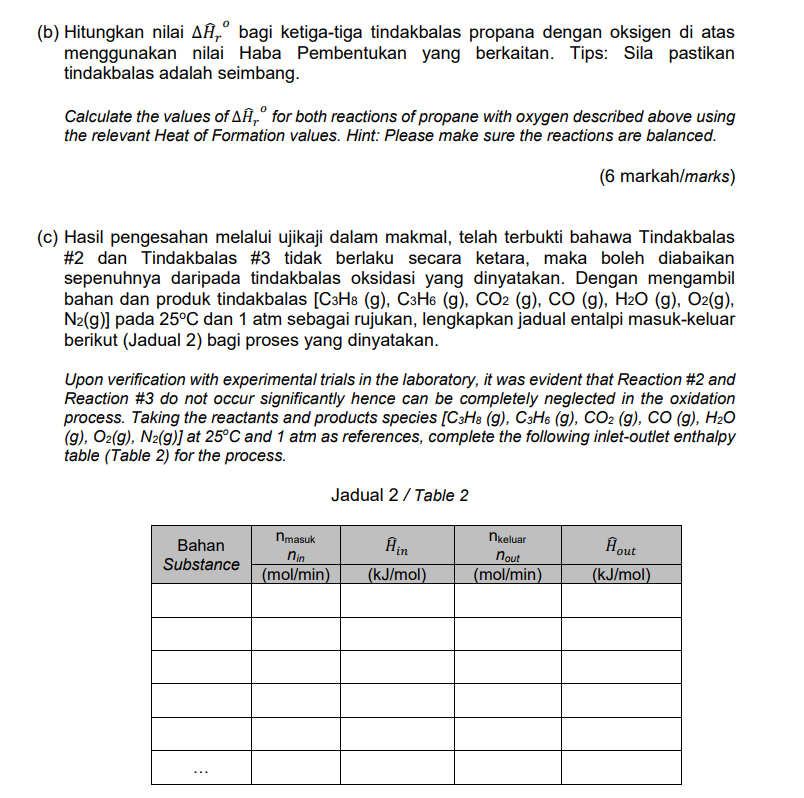

In a continuous process, propane undergoes an oxidation process in air to generate heat. 100 mol/min of propane enters the reactor at 50C while 3000 mol/min of air enters at 400C. All the reaction products exit at 800C. The competing reactions for propane oxidation are as follows: C3Hg (g) + O2(g) CO2 (g) + H20 (v) (Reaction #1) C3Hg (g) + O2(g) CO(g) + H2 (8) (Reaction #2) C3Hg (g) + O2(g) C3H6 (g) + H20 (v) (Reaction #3) 35% of the heat produced is absorbed by water in the boiler tubes located inside the reactor to generate steam at 5 bar. Water enters the boiler tubes at 25C and 1000 kg/h. In this process, you may assume no heat is lost during the heat transfer. (a) Lukiskan gambarajah aliran bagi proses yang dimaksudkan beserta label yang tepat dan lengkap. Draw a flow diagram for the process with accurate and complete labels. (2 markah/marks) (b) Hitungkan nilai A, bagi ketiga-tiga tindakbalas propana dengan oksigen di atas menggunakan nilai Haba Pembentukan yang berkaitan. Tips: Sila pastikan tindakbalas adalah seimbang. Calculate the values of A,for both reactions of propane with oxygen described above using the relevant Heat of Formation values. Hint: Please make sure the reactions are balanced. (6 markah/marks) (c) Hasil pengesahan melalui ujikaji dalam makmal, telah terbukti bahawa Tindakbalas #2 dan Tindakbalas #3 tidak berlaku secara ketara, maka boleh diabaikan sepenuhnya daripada tindakbalas oksidasi yang dinyatakan. Dengan mengambil bahan dan produk tindakbalas [C3H8 (9), C3H6 (g), CO2 (g), CO (g), H2O (9), O2(g), N2(g)] pada 25C dan 1 atm sebagai rujukan, lengkapkan jadual entalpi masuk-keluar berikut (Jadual 2) bagi proses yang dinyatakan. Upon verification with experimental trials in the laboratory, it was evident that Reaction #2 and Reaction #3 do not occur significantly hence can be completely neglected in the oxidation process. Taking the reactants and products species (C3H8 (g), C3H6 (g), CO2 (g), CO (9), H2O (g), O2(g), N2(9)] at 25C and 1 atm as references, complete the following inlet-outlet enthalpy table (Table 2) for the process. Jadual 2 / Table 2 nmasuk nkeluar Bahan Substance Hin nin Hout (kJ/mol) (mol/min) nout (mol/min) (kJ/mol) (d) Hitung jumlah haba yang dipindahkan ke dalam tiub dandang bagi menghasilkan wap air. Seterusnya, tentukan suhu wap air yang terhasil dari proses ini. Nyatakan sebarang andaian yang anda buat dengan jelas. Calculate the amount of heat transferred to the boiler tubes to generate steam. Then determine the final temperature of the steam produced during this process. Clearly state any assumption you made in the calculation. (2 markah/marks) 6/31 In a continuous process, propane undergoes an oxidation process in air to generate heat. 100 mol/min of propane enters the reactor at 50C while 3000 mol/min of air enters at 400C. All the reaction products exit at 800C. The competing reactions for propane oxidation are as follows: C3Hg (g) + O2(g) CO2 (g) + H20 (v) (Reaction #1) C3Hg (g) + O2(g) CO(g) + H2 (8) (Reaction #2) C3Hg (g) + O2(g) C3H6 (g) + H20 (v) (Reaction #3) 35% of the heat produced is absorbed by water in the boiler tubes located inside the reactor to generate steam at 5 bar. Water enters the boiler tubes at 25C and 1000 kg/h. In this process, you may assume no heat is lost during the heat transfer. (a) Lukiskan gambarajah aliran bagi proses yang dimaksudkan beserta label yang tepat dan lengkap. Draw a flow diagram for the process with accurate and complete labels. (2 markah/marks) (b) Hitungkan nilai A, bagi ketiga-tiga tindakbalas propana dengan oksigen di atas menggunakan nilai Haba Pembentukan yang berkaitan. Tips: Sila pastikan tindakbalas adalah seimbang. Calculate the values of A,for both reactions of propane with oxygen described above using the relevant Heat of Formation values. Hint: Please make sure the reactions are balanced. (6 markah/marks) (c) Hasil pengesahan melalui ujikaji dalam makmal, telah terbukti bahawa Tindakbalas #2 dan Tindakbalas #3 tidak berlaku secara ketara, maka boleh diabaikan sepenuhnya daripada tindakbalas oksidasi yang dinyatakan. Dengan mengambil bahan dan produk tindakbalas [C3H8 (9), C3H6 (g), CO2 (g), CO (g), H2O (9), O2(g), N2(g)] pada 25C dan 1 atm sebagai rujukan, lengkapkan jadual entalpi masuk-keluar berikut (Jadual 2) bagi proses yang dinyatakan. Upon verification with experimental trials in the laboratory, it was evident that Reaction #2 and Reaction #3 do not occur significantly hence can be completely neglected in the oxidation process. Taking the reactants and products species (C3H8 (g), C3H6 (g), CO2 (g), CO (9), H2O (g), O2(g), N2(9)] at 25C and 1 atm as references, complete the following inlet-outlet enthalpy table (Table 2) for the process. Jadual 2 / Table 2 nmasuk nkeluar Bahan Substance Hin nin Hout (kJ/mol) (mol/min) nout (mol/min) (kJ/mol) (d) Hitung jumlah haba yang dipindahkan ke dalam tiub dandang bagi menghasilkan wap air. Seterusnya, tentukan suhu wap air yang terhasil dari proses ini. Nyatakan sebarang andaian yang anda buat dengan jelas. Calculate the amount of heat transferred to the boiler tubes to generate steam. Then determine the final temperature of the steam produced during this process. Clearly state any assumption you made in the calculation. (2 markah/marks) 6/31









