Question
In a noninferiority trial, the new treatment is tested to investigate if it is at least similar to an existing therapy in terms of efficacy.
In a noninferiority trial, the new treatment is tested to investigate if it is at least similar to an existing therapy in terms of efficacy. In other words, a noninferiority trial is interested in examining whether a new treatment is not worse than the standard treatment by a prespecified noninferiority margin ?
?0:???versus?1:?>?,H0:???versusH1:?>?, where, ?=?1??2?=?1??2, the difference in means between the experimental treatment (treatment 1) and the standard of care (treatment 2). Rejecting ?0H0 means that the experimental treatment is claimed to be noninferior to (not worse than) the standard treatment. For example if ?=0?=0 and ?=?0.5?=?0.5 then the alternative hypothesis states that the experimental treatment is noninferior to the standard treatment if the mean difference of the outcome in the experimental versus the standard arm is at least -0.5.
Assume that the outcomes of a noninferiority trial are ??1??(?1,?2),?=1,...,?1Yi1?N(?1,?2),i=1,...,n1 in the group receiving the experimental treatment with, and the outcomes in the group receiving the standard treatment are ??2??(?2,?2),?=1,...,?2Yi2?N(?2,?2),i=1,...,n2. In the following questions assume that ?2?2 is known.
(a) Specify the values of the test statistic for which the null hypothesis is rejected (i.e., the rejection region of the test) at level ??.
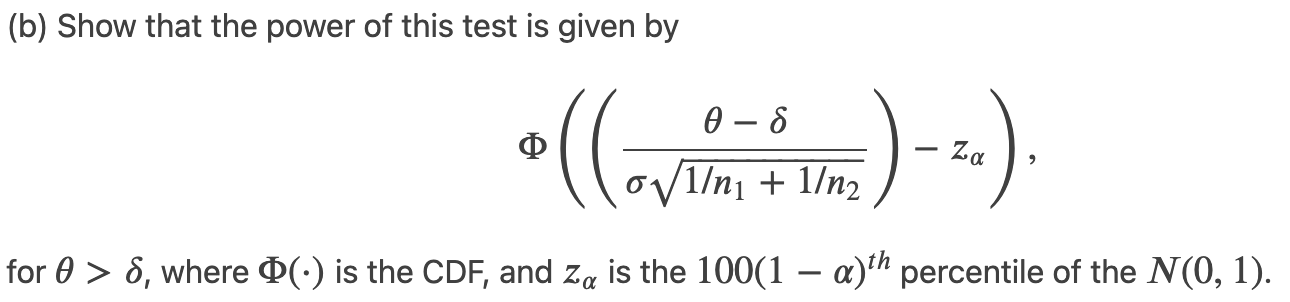
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


