Answered step by step
Verified Expert Solution
Question
1 Approved Answer
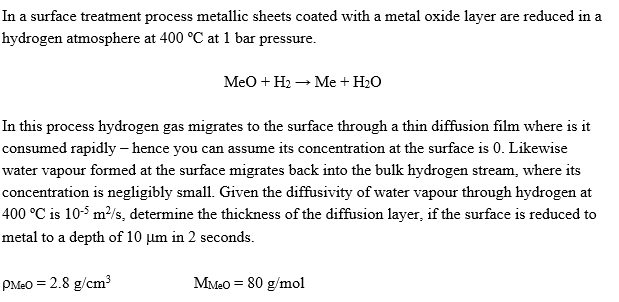
In a surface treatment process metallic sheets coated with a metal oxide layer are reduced in a hydrogen atmosphere at 4 0 0 C at
In a surface treatment process metallic sheets coated with a metal oxide layer are reduced in a
hydrogen atmosphere at at bar pressure.
MeO
In this process hydrogen gas migrates to the surface through a thin diffusion film where is it
consumed rapidly hence you can assume its concentration at the surface is Likewise
water vapour formed at the surface migrates back into the bulk hydrogen stream, where its
concentration is negligibly small. Given the diffusivity of water vapour through hydrogen at
is determine the thickness of the diffusion layer, if the surface is reduced to
metal to a depth of in seconds. Provide diagram for illustration.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


