Answered step by step
Verified Expert Solution
Question
1 Approved Answer
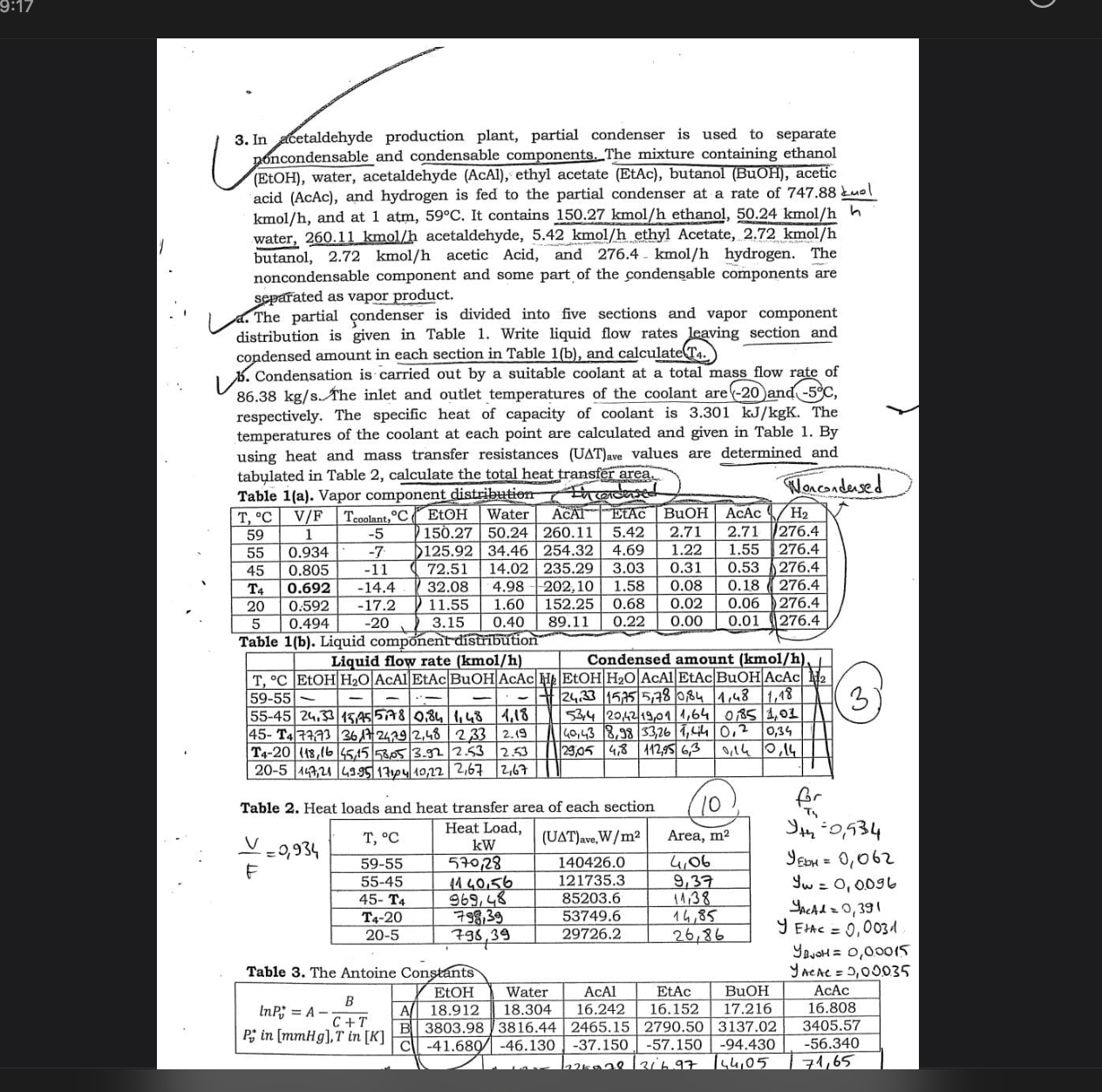
In acetaldehyde production plant, partial condenser is used to separate poncondensable and condensable components. The mixture containing ethanol ( E t O H ) ,
In acetaldehyde production plant, partial condenser is used to separate
poncondensable and condensable components. The mixture containing ethanol
water, acetaldehyde AcAl ethyl acetate EtAc butanol BuOH acetic
acid AcAc and hydrogen is fed to the partial condenser at a rate of mol
kmo and at atm, It contains kmo ethanol, kmo
water, kmo acetaldehyde, kmo ethyl Acetate, kmo
butanol, kmo acetic Acid, and kmo hydrogen. The
noncondensable component and some part of the condensable components are
separated as vapor product.
a The partial condenser is divided into five sections and vapor component
distribution is given in Table Write liquid flow rates leaving section and
condensed amount in each section in Table and calculate
b Condensation is carried out by a suitable coolant at a total mass flow rate of
The inlet and outlet temperatures of the coolant are and
respectively. The specific heat of capacity of coolant is The
temperatures of the coolant at each point are calculated and given in Table By
using heat and mass transfer resistances values are determined and
tabulated in Table calculate the total heat transfer area.
Table a Vapor component distribution Noncondersed
Table Heat loads and heat transfer area of each section
for
Table The Antoine Constants
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


