Question
In an absorption column, NH3 is absorbed with water from the NH3 air mixture at 25 C and 101.3 kPa pressure. The water fed from
In an absorption column, NH3 is absorbed with water from the NH3 air mixture at 25 C and 101.3 kPa pressure. The water fed from the tower top contains 1% NH3 as mole percent. The flow rate of the NH3 air mixture fed from the tower base and containing 30% NH3 as mole percent is 800 kmol/h. The air leaving the column contains 2% NH3 as mole percent. Since the water leaving the tower at the end of the process is desired to contain 15% NH3 as a mole percent;
a) Find the percent recovery of NH3. b) Calculate how much the flow rate of the water sent from the top of the tower is more than the minimum amount. c) Derive the operating curve equation.
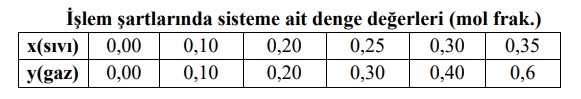 lem artlarnda sisteme ait denge deerleri (mol frak.)
lem artlarnda sisteme ait denge deerleri (mol frak.) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


