Question
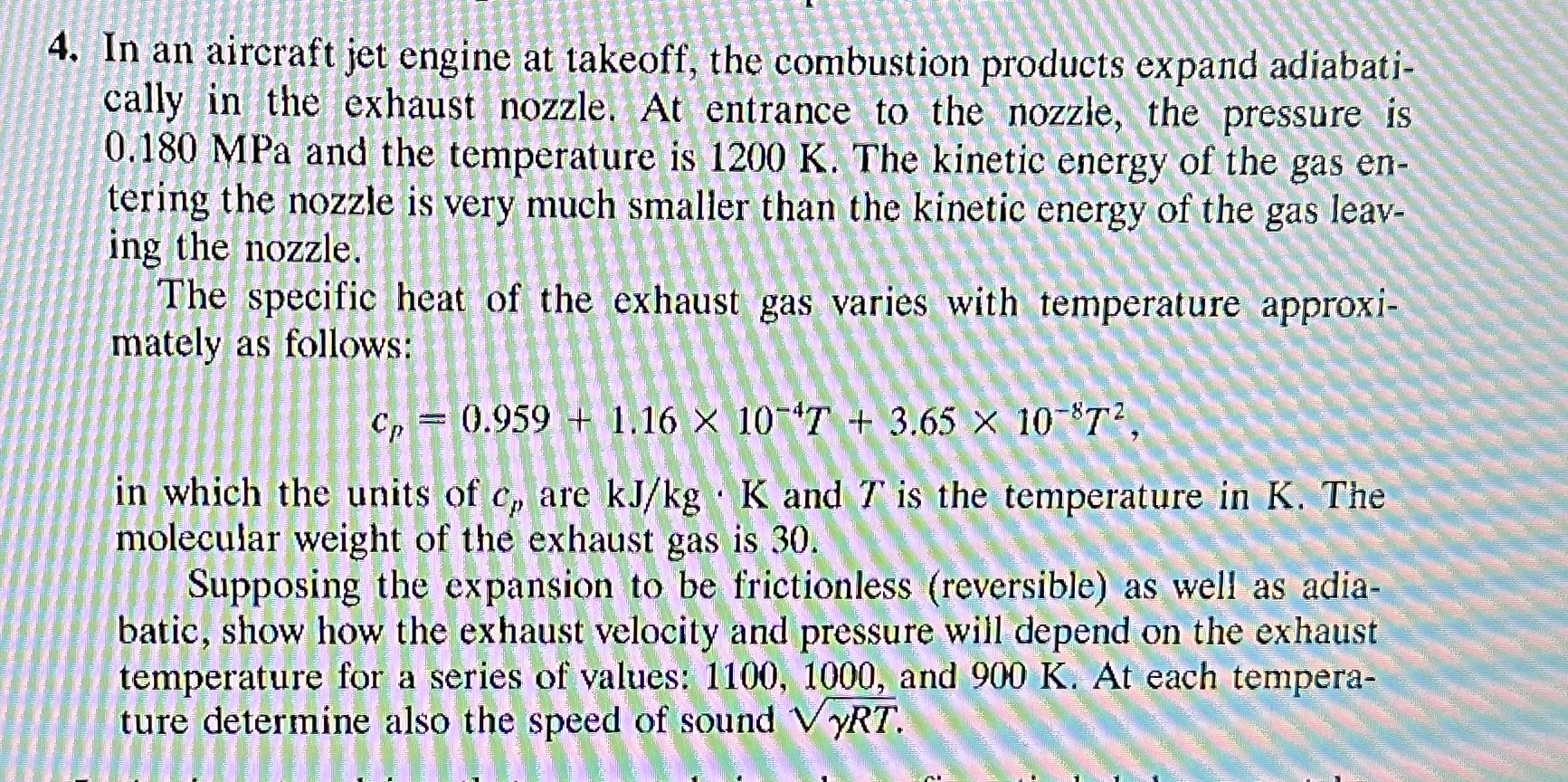
In an aircraft jet engine at takeoff, the combustion products expand adiabatically in the exhaust nozzle. At entrance to the nozzle, the pressure is 0.180MPa
In an aircraft jet engine at takeoff, the combustion products expand adiabatically in the exhaust nozzle. At entrance to the nozzle, the pressure is
0.180MPaand the temperature is
1200K. The kinetic energy of the gas entering the nozzle is very much smaller than the kinetic energy of the gas leaving the nozzle.\ The specific heat of the exhaust gas varies with temperature approximately as follows:\
c_(p)=0.959+1.16\\\\times 10^(-4)T+3.65\\\\times 10^(-8)T^(2),\ in which the units of
c_(p)are
k(J)/(k)g*Kand
Tis the temperature in
K. The molecular weight of the exhaust gas is 30 .\ Supposing the expansion to be frictionless (reversible) as well as adiabatic, show how the exhaust velocity and pressure will depend on the exhaust temperature for a series of values: 1100,1000 , and
900K. At each temperature determine also the speed of sound
\\\\sqrt(\\\\gamma RT).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


