Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In an electron microscope, a beam of high energy electrons can knock out core electrons from atoms in the sample. The subsequent relaxation of
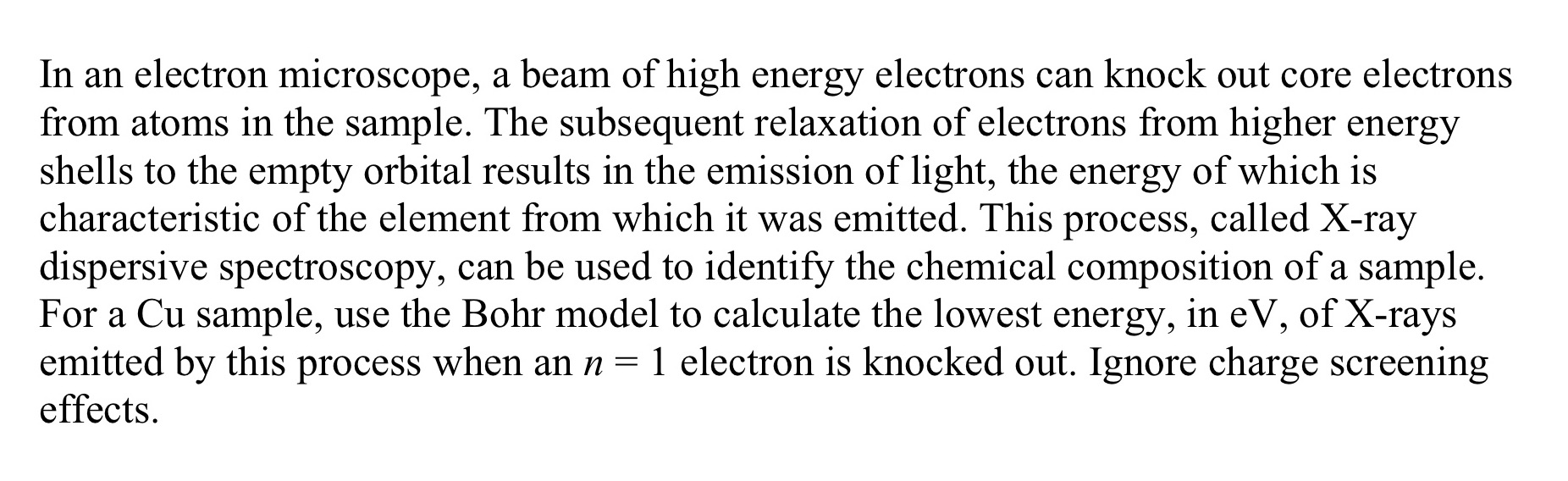
In an electron microscope, a beam of high energy electrons can knock out core electrons from atoms in the sample. The subsequent relaxation of electrons from higher energy shells to the empty orbital results in the emission of light, the energy of which is characteristic of the element from which it was emitted. This process, called X-ray dispersive spectroscopy, can be used to identify the chemical composition of a sample. For a Cu sample, use the Bohr model to calculate the lowest energy, in eV, of X-rays emitted by this process when an n = 1 electron is knocked out. Ignore charge screening effects.
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
In the Bohr model the energy levels of an electron in a hydrogenlik...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


