Answered step by step
Verified Expert Solution
Question
1 Approved Answer
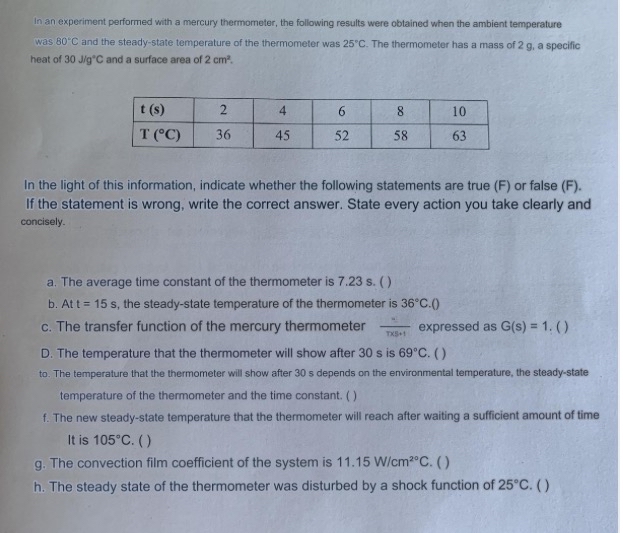
In an experiment performed with a mercury thermometer, the following results were obtained when the ambient temperature was 8 0 C and the steady -
In an experiment performed with a mercury thermometer, the following results were obtained when the ambient temperature was and the steadystate temperature of the thermometer was The thermometer has a mass of a specific heat of and a surface area of
table
In the light of this information, indicate whether the following statements are true F or false F If the statement is wrong, write the correct answer. State every action you take clearly and concisely.
a The average time constant of the thermometer is
b At the steadystate temperature of the thermometer is
c The transfer function of the mercury thermometer expressed as
D The temperature that the thermometer will show after is
to The temperature that the thermometer will show after depends on the environmental ternperature, the steadystate temperature of the thermometer and the time constant.
f The new steadystate temperature that the thermometer will reach after waiting a sufficient amount of time It is
g The convection film coefficient of the system is
h The steady state of the thermometer was disturbed by a shock function of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


