Question
In an experiment to determine the heat of combustion of methanol (CH3OH) a student used a set up like the one shown in the diagram
In an experiment to determine the heat of combustion of methanol (CH3OH) a student used a set up like the one shown in the diagram below. Study the set- up and the data below it and answer the questions that follows
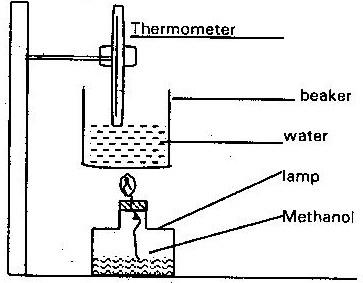
Volume of water = 500cm3
Final temperature of water = 27.00C
Initial temperature of water = 20.00C
Final mass of lamp + methanol = 22.11g
Initial mass of lamp + methanol = 22.98g
Density of water = 1.0/ cm3
Heat change = mass x temperature x 4.2j/g/C
(a) Write an equation for the combustion of methanol ( 1 mk)
(b) Calculate
(i) The number of moles of methanol used in the experiment (C=12), (O= 16) (H=1) ( 2 mks)
(ii) Heat change in this experiment
(iii) The heat of combustion per mole of methanol ( 2 mks)
Thermometer www.dow beaker water "lamp Methanol
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
a 2CH 3 OH 3O 2 2CO 2 4H 2 O b i The number of moles used by ethanol can be determined by mass of me...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


