Answered step by step
Verified Expert Solution
Question
1 Approved Answer
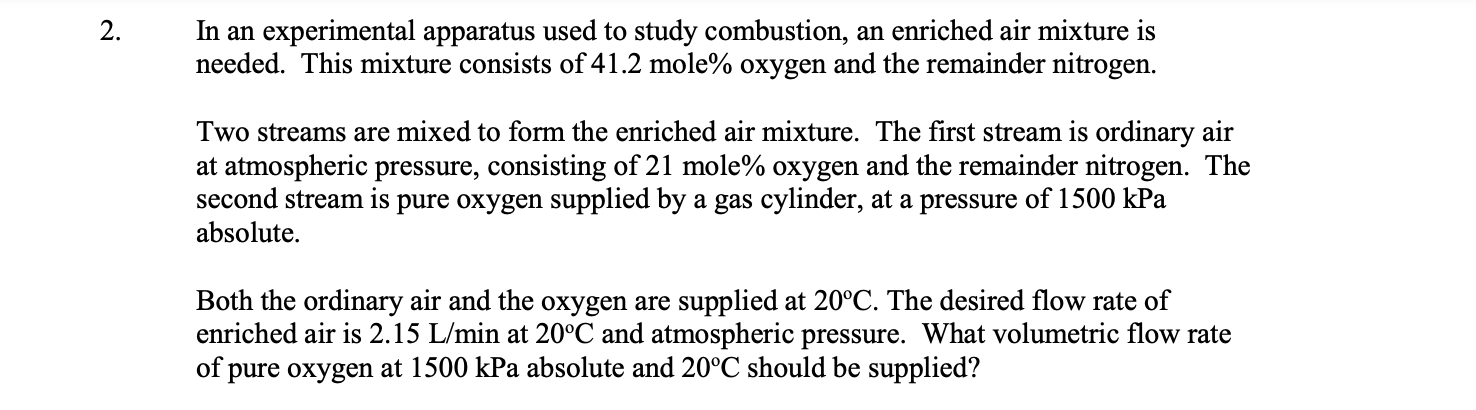
In an experimental apparatus used to study combustion, an enriched air mixture is needed. This mixture consists of 4 1 . 2 mole % oxygen
In an experimental apparatus used to study combustion, an enriched air mixture is
needed. This mixture consists of mole oxygen and the remainder nitrogen.
Two streams are mixed to form the enriched air mixture. The first stream is ordinary air
at atmospheric pressure, consisting of mole oxygen and the remainder nitrogen. The
second stream is pure oxygen supplied by a gas cylinder, at a pressure of kPa
absolute
Both the ordinary air and the oxygen are supplied at The desired flow rate of
enriched air is at and atmospheric pressure. What volumetric flow rate
of pure oxygen at kPa absolute and should be supplied?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


