Question
In an ideal Hampson-Linde cycle, no heat is lost to the environment, i.e. it is assumed that the enthalpy remains constant in the combination of
In an ideal Hampson-Linde cycle, no heat is lost to the environment, i.e. it is assumed that the enthalpy remains constant in the combination of the throttle valve and the regenerative heat exchanger. In other words, the total enthalpy of the circulating medium entering the choke is the same as the circulating medium leaving the heat exchanger. a) It is assumed that x is the liquefiable fraction of the circulating medium in each revolution. Prove that 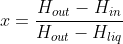 ,where the molar enthalpies are Hin for the compressed gas entering the heat exchanger, Hout for the low-pressure gas leaving the heat exchanger, and Hliq for the liquefied circulating medium. b) Using the attached table for nitrogen, determine how much nitrogen is liquefied in each cycle of the Hampson-Linden evaporator when the operating pressures of the apparatus are 1 and 100 bar, with a gas supply temperature of 300 K. Suppose that the heat exchanger works perfectly, so that the temperature of the low-pressure gas when leaving the heat exchanger is the same as that of the compressed gas going to the choke. c) How does the situation change when the gas supply temperature is 200 K?
,where the molar enthalpies are Hin for the compressed gas entering the heat exchanger, Hout for the low-pressure gas leaving the heat exchanger, and Hliq for the liquefied circulating medium. b) Using the attached table for nitrogen, determine how much nitrogen is liquefied in each cycle of the Hampson-Linden evaporator when the operating pressures of the apparatus are 1 and 100 bar, with a gas supply temperature of 300 K. Suppose that the heat exchanger works perfectly, so that the temperature of the low-pressure gas when leaving the heat exchanger is the same as that of the compressed gas going to the choke. c) How does the situation change when the gas supply temperature is 200 K?

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


