Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In chemical kinetics, it is well understood how temperature influences the rates of collisions between reactants. To slow down this interaction, the ambient temperature
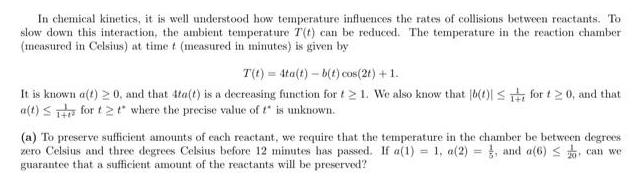
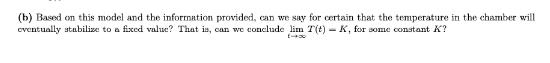
In chemical kinetics, it is well understood how temperature influences the rates of collisions between reactants. To slow down this interaction, the ambient temperature T(t) can be reduced. The temperature in the reaction chamber (measured in Celsius) at time t (measured in minutes) is given by T(t)=4ta(t)-b(t) cos(2t) + 1. It is known a(t) 0, and that 4ta(t) is a decreasing function for t21. We also know that [b(1)| for 120, and that a(t) for t2t where the precise value of t* is unknown. (a) To preserve sufficient amounts of each reactant, we require that the temperature in the chamber be between degrees zero Celsius and three degrees Celsius before 12 minutes has passed. If a(1) = 1, a(2), and a(6) can we 20 guarantee that a sufficient amount of the reactants will be preserved? (b) Based on this model and the information provided, can we say for certain that the temperature in the chamber will eventually stabilize to a fixed value? That is, can we conclude lim T(t)- K, for some constant K? 140
Step by Step Solution
★★★★★
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
To determine if a sufficient amount of reactants will ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


