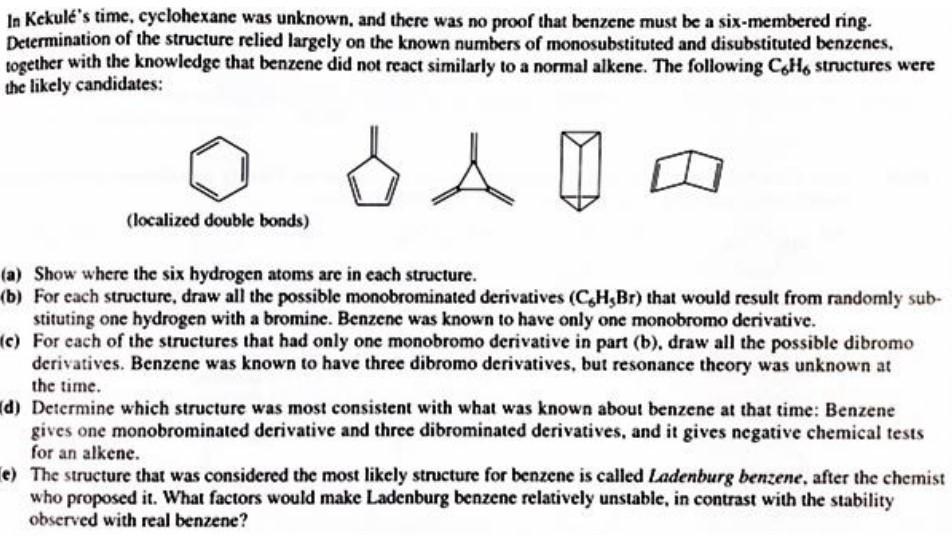
In Kekule's time, cyclohexane was unknown, and there was no proof that benzene must be a six-membered ring. Determination of the structure relied largely on the known numbers of monosubstituted and disubstituted benzenes. together with the knowledge that benzene did not react similarly to a normal alkene. The following C6H6 structures were the likely candidates: a) Show where the six hydrogen atoms are in each structure. b) For each structure, draw all the possible monobrominated derivatives (C6H3Br) that would result from randomly substituting one hydrogen with a bromine. Benzene was known to have only one monobromo derivative. c) For each of the structures that had only one monobromo derivative in part (b), draw all the possible dibromo derivatives. Benzene was known to have three dibromo derivatives, but resonance theory was unknown at the time. d) Determine which structure was most consistent with what was known about benzene at that time: Benzene gives one monobrominated derivative and three dibrominated derivatives, and it gives negative chemical tests for an alkene. e) The structure that was considered the most likely structure for benzene is called Ladenburg benzene, after the chemist who proposed it. What factors would make Ladenburg benzene relatively unstable, in contrast with the stability observed with real benzene? In Kekule's time, cyclohexane was unknown, and there was no proof that benzene must be a six-membered ring. Determination of the structure relied largely on the known numbers of monosubstituted and disubstituted benzenes. together with the knowledge that benzene did not react similarly to a normal alkene. The following C6H6 structures were the likely candidates: a) Show where the six hydrogen atoms are in each structure. b) For each structure, draw all the possible monobrominated derivatives (C6H3Br) that would result from randomly substituting one hydrogen with a bromine. Benzene was known to have only one monobromo derivative. c) For each of the structures that had only one monobromo derivative in part (b), draw all the possible dibromo derivatives. Benzene was known to have three dibromo derivatives, but resonance theory was unknown at the time. d) Determine which structure was most consistent with what was known about benzene at that time: Benzene gives one monobrominated derivative and three dibrominated derivatives, and it gives negative chemical tests for an alkene. e) The structure that was considered the most likely structure for benzene is called Ladenburg benzene, after the chemist who proposed it. What factors would make Ladenburg benzene relatively unstable, in contrast with the stability observed with real benzene







