Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In Matlab The molecular weight (MW) of any compound is the sum of the products the number atoms of each element (Z) and the atomic
In Matlab 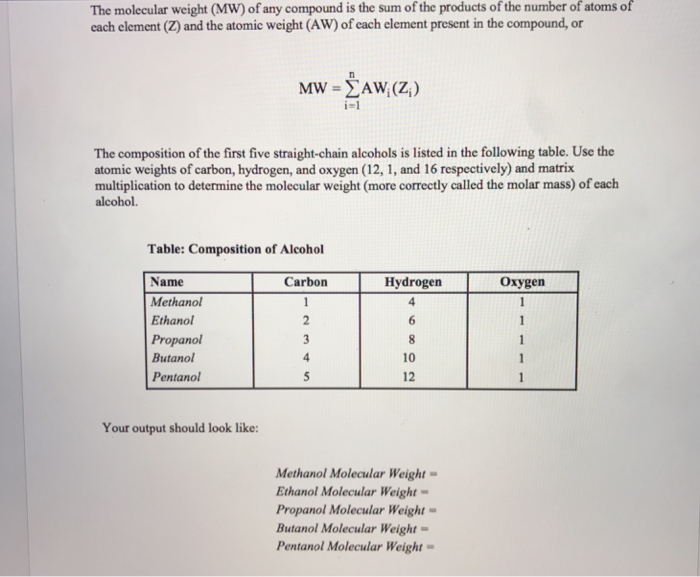
The molecular weight (MW) of any compound is the sum of the products the number atoms of each element (Z) and the atomic weight (AW) of each element present in the compound, or of of i-1 The composition of the first five straight-chain alcohols is listed in the following table. Use the atomic weights of carbon, hydrogen, and oxygen (12, 1, and 16 respectively) and matrix multiplication to determine the molecular weight (more correctly called the molar mass) of each alcohol. Table: Composition of Alcohol Hydrogen 4 Carbon Oxygen Name Methanol Ethanol Propanol Butanol Pentanol 10 12 Your output should look like: Methanol Molecular Weight Ethanol Molecular Weight Propanol Molecular Weight Butanol Molecular Weight Pentanol Molecular Weight 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


