Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In order to oxidize A (gas), $25A and $75 air mixture is fed to the flow reactor with 900s/s volumetric flow rate. Reaction is carried
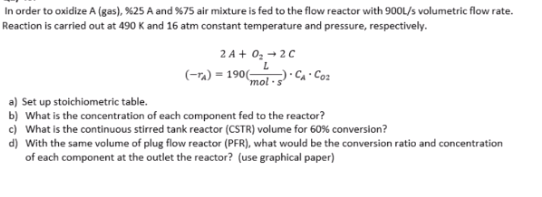 In order to oxidize A (gas), $25A and $75 air mixture is fed to the flow reactor with 900s/s volumetric flow rate. Reaction is carried out at 490K and 16 atm constant temperature and pressure, respectively. 2A+O22C(rA)=190(molsL)CACo2 a) Set up stoichiometric table. b) What is the concentration of each component fed to the reactor? c) What is the continuous stirred tank reactor (CSTR) volume for 60% conversion? d) With the same volume of plug flow reactor (PFR), what would be the conversion ratio and concentration of each component at the outlet the reactor? (use graphical paper)
In order to oxidize A (gas), $25A and $75 air mixture is fed to the flow reactor with 900s/s volumetric flow rate. Reaction is carried out at 490K and 16 atm constant temperature and pressure, respectively. 2A+O22C(rA)=190(molsL)CACo2 a) Set up stoichiometric table. b) What is the concentration of each component fed to the reactor? c) What is the continuous stirred tank reactor (CSTR) volume for 60% conversion? d) With the same volume of plug flow reactor (PFR), what would be the conversion ratio and concentration of each component at the outlet the reactor? (use graphical paper) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


