Question
In Prof. Patersons synthesis, the thioester (1) was deprotonated to generate an enolate (2) with defined stereochemistry, which then participates in an aldol reaction with
In Prof. Paterson’s synthesis, the thioester (1) was deprotonated to generate an enolate (2) with defined stereochemistry, which then participates in an aldol reaction with aldehyde (3) to give the product (4)
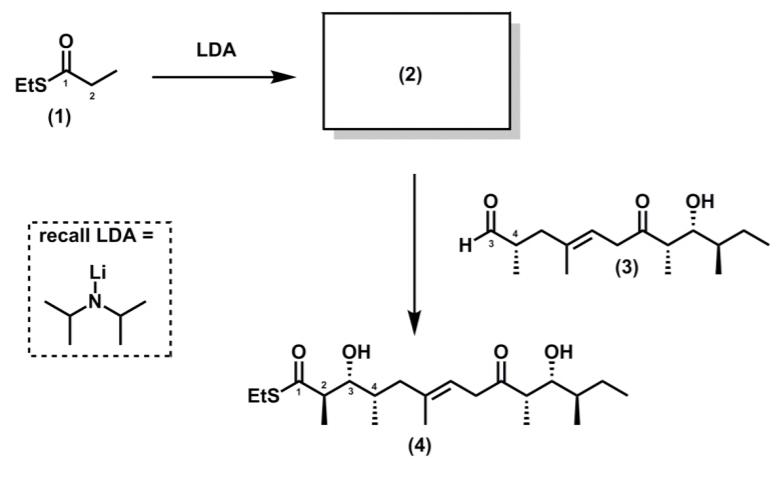
(a) Suggest a structure for enolate (2)
(b) Using cyclic deprotonation models, justify why the enolate (2) has a particular stereochemistry (E or Z).
(c) Using your knowledge of Felkin-Anh control, rationalize why compound (4) has a syn stereochemical relationship between the stereogenic centres labelled 3 and 4.
(d) Using 3D representations, invoke a Zimmerman structure to account for why compound (4) has the anti stereochemical relationship between the stereogenic centres labelled 2 and 3.
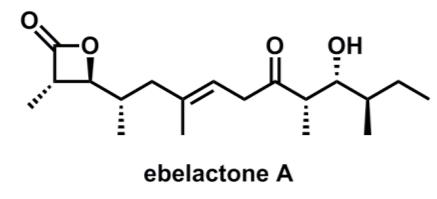
ebelactone A OH
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Intellectual property IP pertains to any original creation of the human intellect such as artistic l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


