Question
During Prof. K. C. Nicolaous synthesis of the oestradiol analogue, methylcyclopentanone (5) underwent a reaction cascade to give a stereodefined product (7). (a) Suggest a
During Prof. K. C. Nicolaou’s synthesis of the oestradiol analogue, methylcyclopentanone (5) underwent a reaction cascade to give a stereodefined product (7).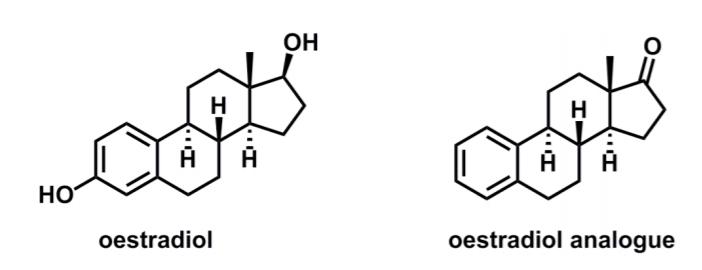
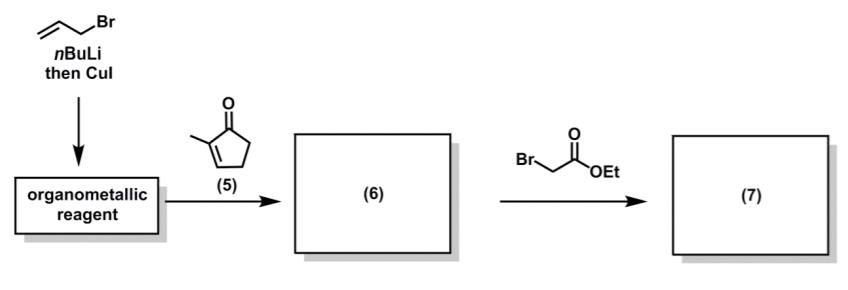
(a) Suggest a structure for the organometallic reagent. 2 marks
(b) Suggest a structure for the intermediate (6). 1 mark
(c) Using curly arrow notation, draw out the mechanism for the conversion of compound (5) into intermediate (6). 1 mark
(d) Suggest a structure for the compound (7). 2 mark
(e) Using curly arrow notation, draw out the mechanism for the conversion of intermediate (6) into compound (7). 1 mark
(f) Use 3-dimensional structures to rationalize the relative stereochemistry of compound (6). 2 marks
(g) Is the synthesis of compound (7) enantioselective, racemic, or achiral? 1 mark
H H HO oestradiol oestradiol analogue
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


