Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In step 4, how does it go from square root of 1.6x10^-5 to right below that 4.0x10^-3? in other words, How did they get 4.0x10^-3
In step 4, how does it go from square root of 1.6x10^-5 to right below that 4.0x10^-3?
Review the neps in this calculation by iewriting them by hind Dea't wig acy steps. One of your futre quizss will be like Problen I ar Preblen 2 with differtet sember in it. Protlem 1: For a 0.85M solutice of a weak, mohoprotic acid with a Ki of 1.5xi05, alculate [H'] (he proboe concentrition) and the pH. Sec2 Kii=[HA]LHUIXIAI 1.8109=0.85x3.x Step 3: Assine is imall mach the 0.35=0.856. DN THa Naxt 5 stars, tise =2400 ) Step 4: 1,9x10=0.55x2 (1.9107)(0.85)=x2 1. 5.101=x2 4.0z103Max=[H1] xDOES EOUALTH SOUsE - THRTE Socp 5: pift =log[H] pll =2% in other words, How did they get 4.0x10^-3 from square root of 1.6x10^-5? 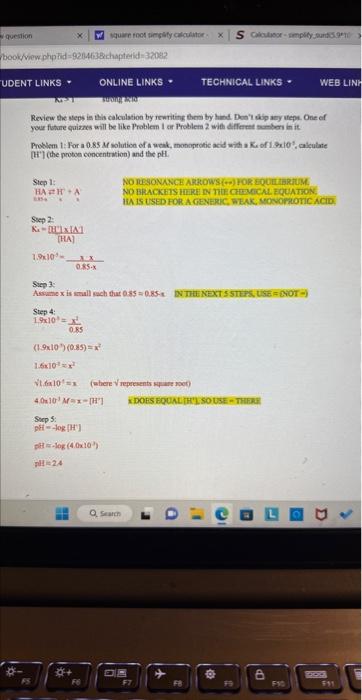

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


