Answered step by step
Verified Expert Solution
Question
1 Approved Answer
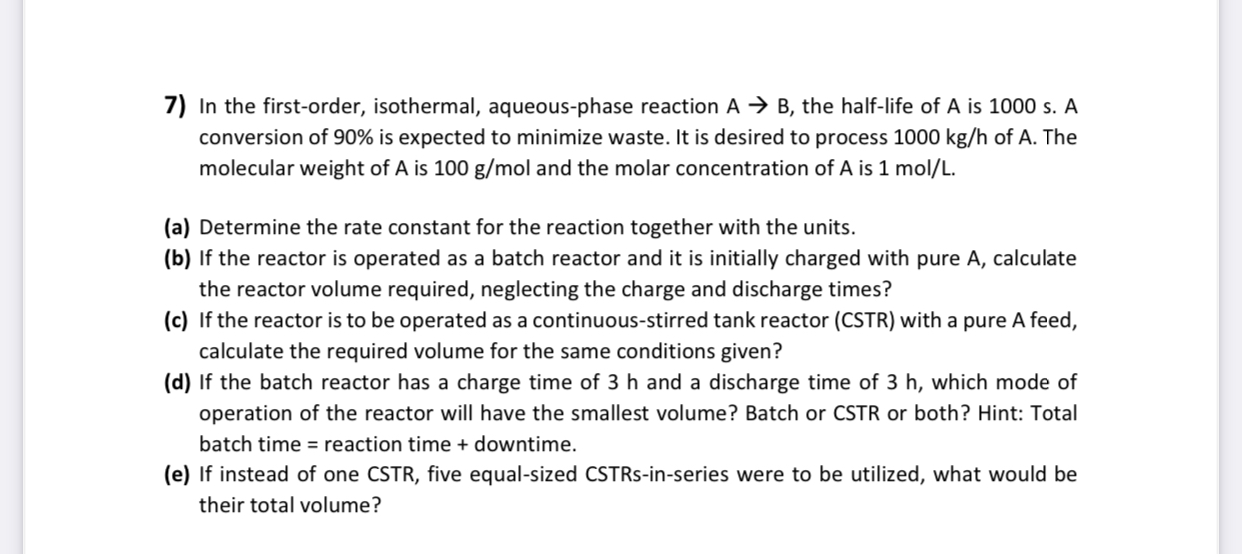
In the first - order, isothermal, aqueous - phase reaction A B , the half - life of A is 1 0 0 0 s
In the firstorder, isothermal, aqueousphase reaction the halflife of is A conversion of is expected to minimize waste. It is desired to process of The molecular weight of is and the molar concentration of is
a Determine the rate constant for the reaction together with the units.
b If the reactor is operated as a batch reactor and it is initially charged with pure A calculate the reactor volume required, neglecting the charge and discharge times?
c If the reactor is to be operated as a continuousstirred tank reactor CSTR with a pure A feed, calculate the required volume for the same conditions given?
d If the batch reactor has a charge time of and a discharge time of which mode of operation of the reactor will have the smallest volume? Batch or CSTR or both? Hint: Total batch time reaction time downtime
e If instead of one CSTR five equalsized CSTRsinseries were to be utilized, what would be their total volume?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


